Zilretta - a New Drug FDA Approved for Osteoarthritis of the Knee Save
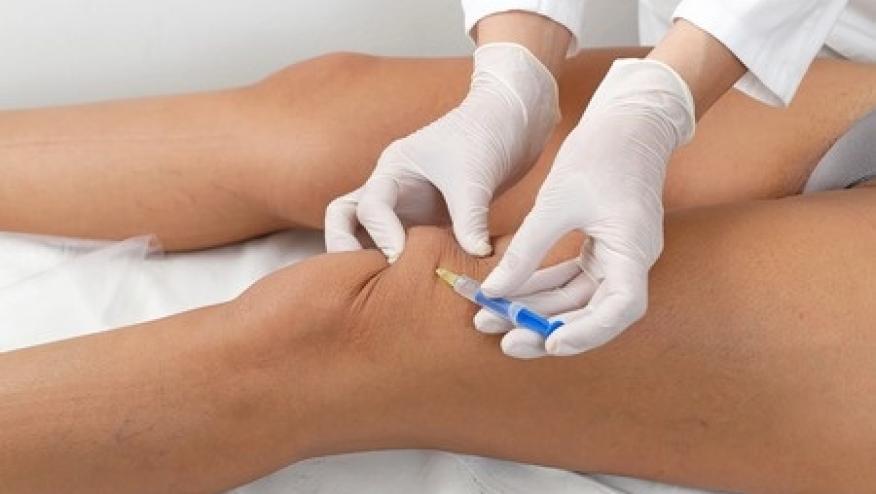
Flexion Therapeutics announced friday that the U.S. Food and Drug Administration (FDA) has approved its osteoarthritis (OA) of the knee injectable steroid drug Zilretta with the indication of moderate-to-severe knee pain.
Zilretta is the commonly used triamcinolon acetonide combined with a drug delivery system designed to provide extended pain relief over three months.
It will be priced at $570 per dose. The company claims the therapy to be cost effective based on a comparative analysis against diclofenac or hyaluronic acid (HA) injections presented earlier this year.
They claim that Zilretta produced an average QALY gain from baseline of 0.189 per six months, which suggests a greater improvement in quality of life compared with the average values for conventional care (0.030), diclofenac (0.078) and HA injections (0.109). Zilretta yielded a cost per QALY estimate of $3,201, compared with cost per QALY estimates for conventional care ($10,717), diclofenac ($2,708) and an average HA treatment course ($13,389). (Citation source: https://buff.ly/2fW2CgE)
In a nearly 500-patient Phase 3 study, Zilretta significantly lowered pain intensity scores compared to placebo three months following administration. A secondary analysis comparing Zilretta to an active control of immediate-release triamcinolone acetonide showed numerically superior reduction but failed to hit statistical significance.
Flexion, though, is also touting Zilretta's muted effect on blood sugar levels. Spikes in blood glucose levels are a known potential side effect of corticosteroids. Roughly one-fifth of OA patients have type 2 diabetes, meaning a pain treatment with a reduced risk of increasing blood sugar would likely be viewed more favorably by physicians.
A small study of Zilretta with type 2 diabetes patients showed treatment with the drug led to a significantly smaller increase in blood glucose levels than other formulations of triamcinolone acetonide.
The market for osteoarthritis drugs is expected to increase from $1.6 billion in 2016 to $3.5 billion by 2026, according to GlobalData.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.