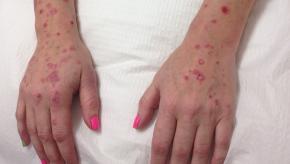
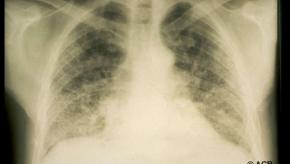
Mortality in Psoriatic Arthritis
The Annals of Rheumatic Disease reports that mortality risk in psoriatic arthritis (PsA) is about 10% higher than in the general population, primarily from excess comorbidity and with increased risks in women, with longer disease durations.