IL-23
The arsenal of therapeutics for psoriatic arthritis continues to increase. Risankizumab is an IL-23 inhibitor to the p19 subunit, currently being investigated for treatment of psoriatic arthritis. I’…

3 years 4 months ago
KEEPsAKE trial 1 (DMARD-IR) and 2 (DMARD/biologic-IR) on risankizumab (IL23i) for PsA:
👉some benefits of risankizumab for axial disease
👉no data regarding bowel disease
👉pts did remain on background DMARDS, steroids.
(2/2)

3 years 4 months ago
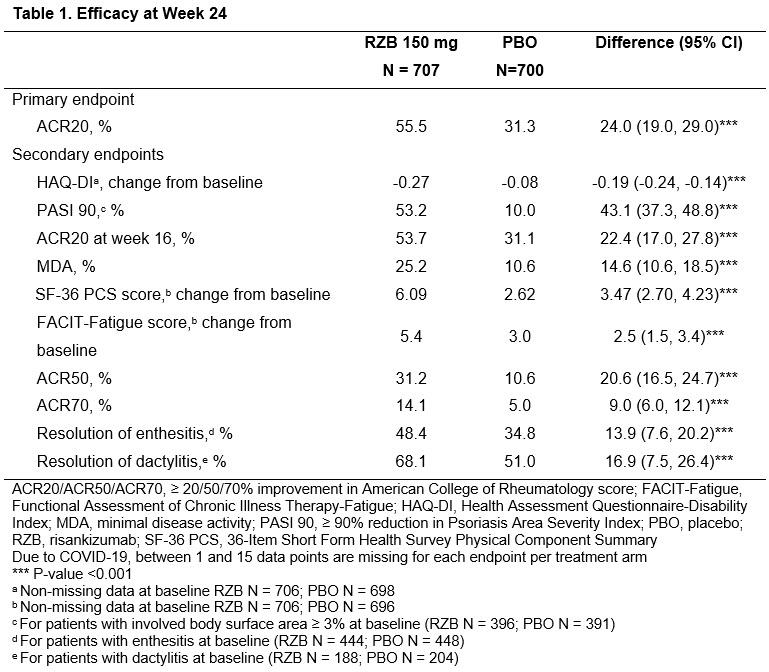
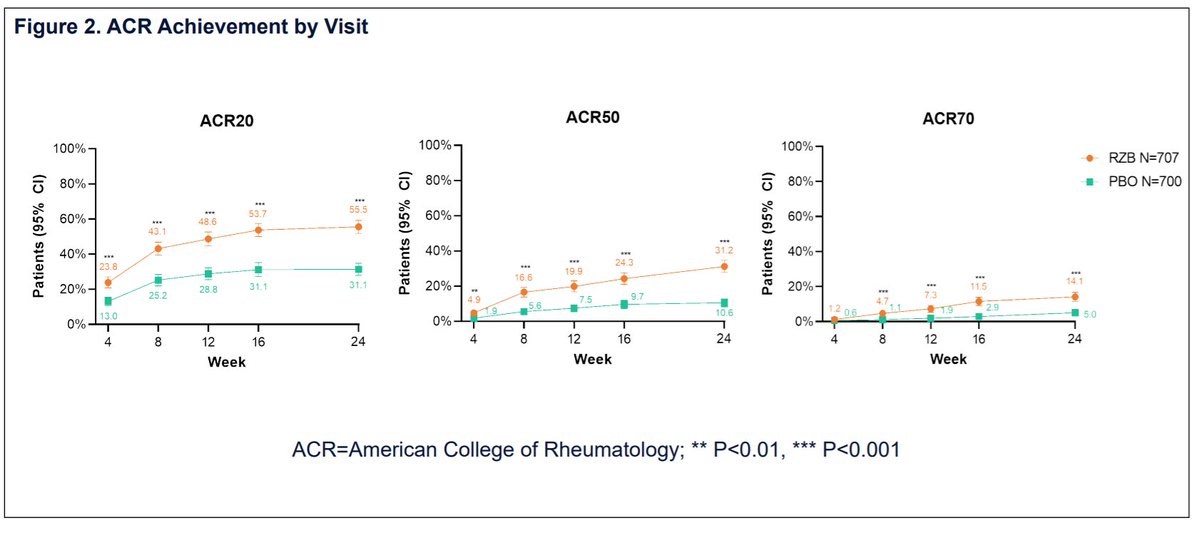
Risankizumab (IL-23i) KEEPsAKE 1 and 2 (phase 3) studies for PsA treatment
⬆️ACR20 response compared to pbo
⬆️secondary endpoints
⭐️no new safety signals
Abs#453
#ACR21 #ACRBest @RheumNow
https://t.co/7bwvIPMz38 https://t.co/TJaSqwipa5

3 years 4 months ago
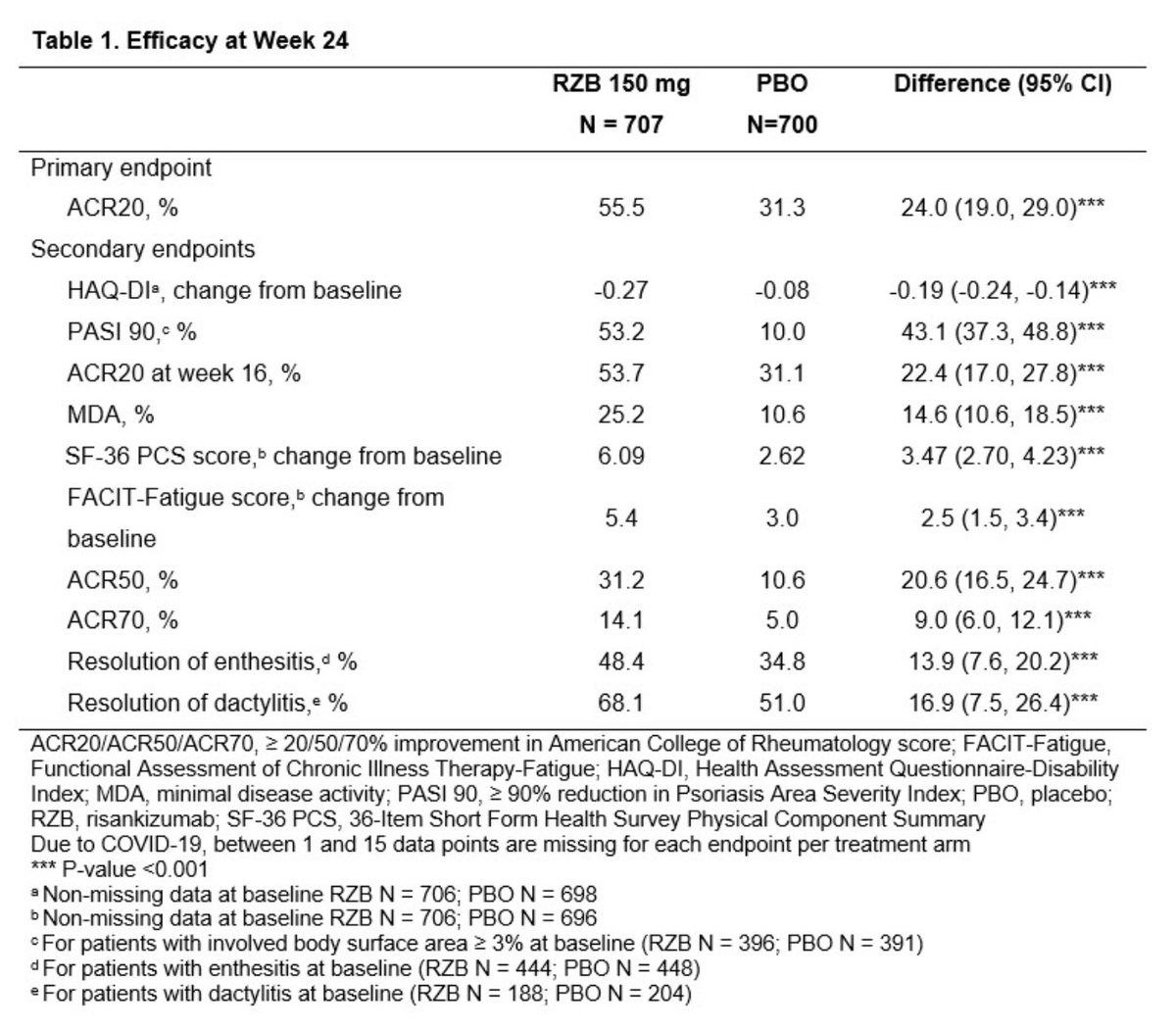
#ACR21 Abst#0453. KEEPsAKE: Risankizumab (Skyrizi) monoclonal Ab inhibits IL23 by binding p19.
▶️150 mg RZB vs Placebo
⭐️RZB: higher ACR20/50/70, PASI90, HAQ-DI
⭐️Resolution of enthesitis, dactylitis. Improve fatigue, fxn
⭐️No safety signal
https://t.co/bqQJ4Zr7o5 @Rheumnow https://t.co/2ZfGRs2GUd

3 years 4 months ago
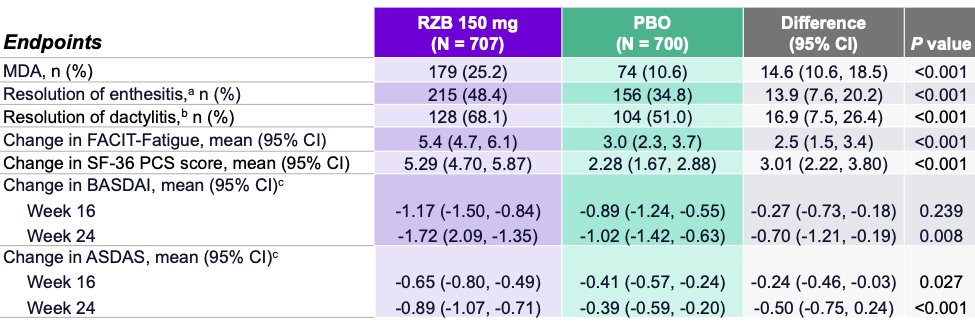
KEEPsAKE trial 1 (DMARD-IR) and 2 (DMARD/biologic-IR) on risankizumab (IL23i) for PsA:
👉phase 3 DBRPCT 24 weeks
👉150 mg week 0,4, 16 weeks
👉improved ACR20,ACR50,ACR70
👉improved PASI90
👉improved HAQ-DI
👉no increased safety signals
#ACR21 Abstr#0438 #Plenary @rheumnow https://t.co/ioIBXgV4Hi

3 years 4 months ago
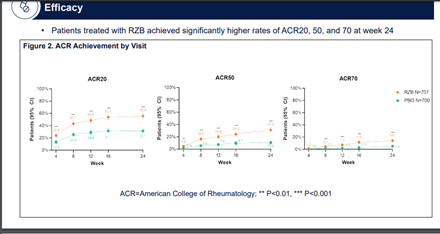
Promising results of Risankizumab for #psoriaticarthritis
🔸Pts significantly achieved higher rates of ACR20, 50 and 70 response at wk 24 vs. placebo
🔸Well tolerated and showed no safety signals
@RheumNow #ACR21 #ACRBest abs0453 https://t.co/TDSLEgF68g


3 years 4 months ago
Abst0453 #ACR21 @RheumNow integrated phase 2/3 trial results of il23 inhibitor Risankizumab (RZB) in PsA: significant improvement ACR20 at Week 24 (RZB 55.5% vs PBO 31.3%, P < 0.001), secondary endpoint met at HAQ DI, PASI, fatigue score& enthesitis. no new safety signals https://t.co/ZAVgswoXCf

3 years 4 months ago
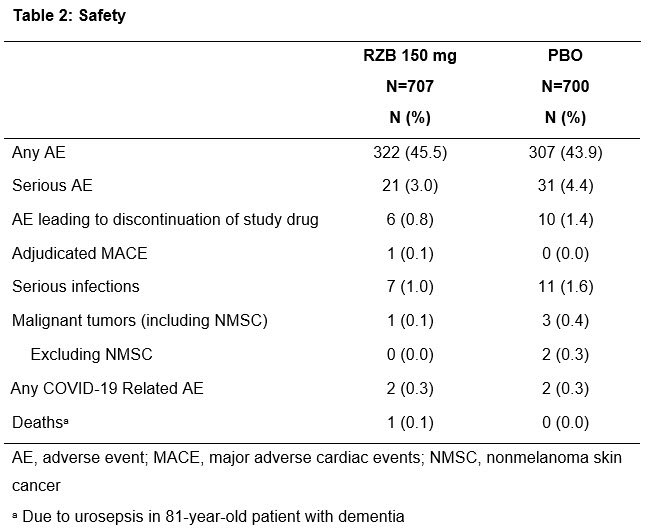
RZB tx resulted in statistically greater improvements in signs/ symptoms of PsA vs PBO and was well tolerated w/ no new safety signals. Plenary Abs 0453 #ACR21 #RheumNow #ACRbest @RheumNow https://t.co/c9RJdWlmpO https://t.co/MDbdAhdnIy

3 years 4 months ago
Dr Oster presenting on IL-23 inhibitor Risankizumab in PsA. No surprises here, it works. We need comparative efficacy trials! Abstr#0453 #ACR21 @RheumNow https://t.co/mj8TeCqEcg













 Poster Hall
Poster Hall