JAK/TYK2

Dr. Rachel Tate uptoTate
3 years 2 months ago
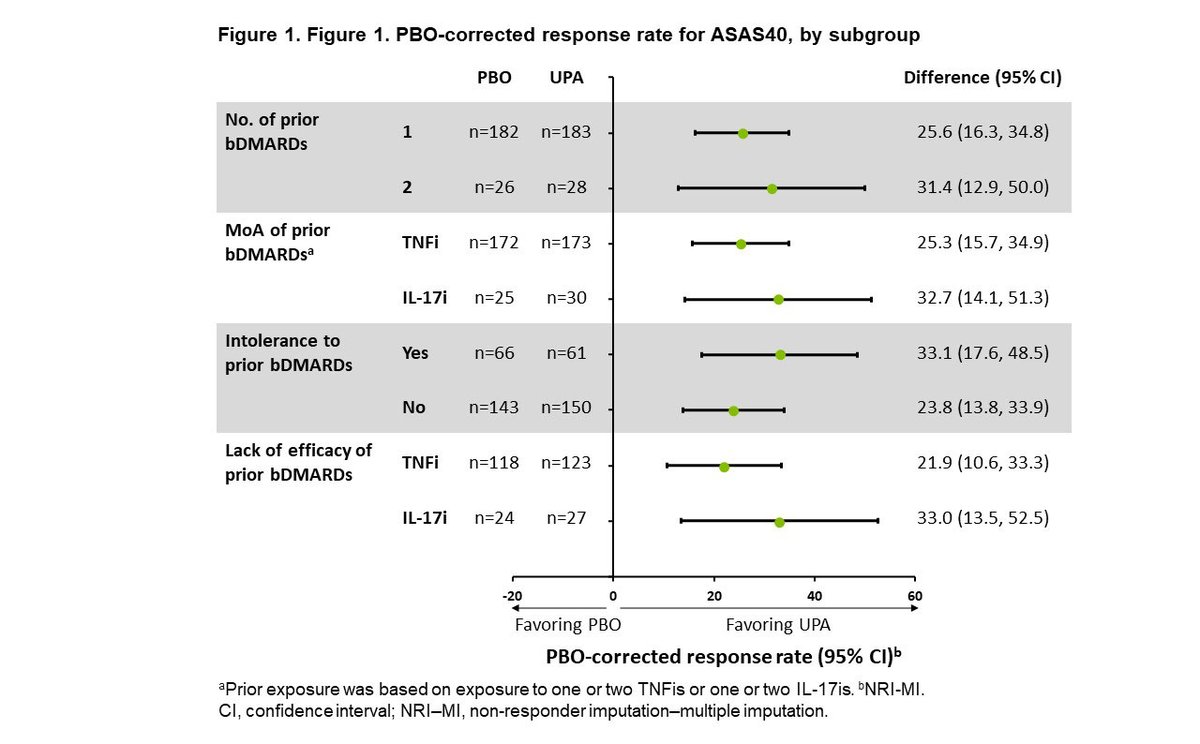
SELECT-AXIS 2 subgroup analysis. UPA demonstrated improved efficacy vs PBO at Wk 14 across all evaluated subgroups of pts with bDMARD-IR AS. No new safety signals. @XBaraliakos et al, Abs 0414 #ACR22 https://t.co/lTfm68XlEY https://t.co/PaWdrOljMO
The RheumNow faculty reporters have been scouring the meeting and online presentations to find the best abstracts from ACR22. Here are some of their choice abstracts reported today on day 1 of ACR 2022 (#ACRbest).

Dr. Rachel Tate uptoTate
3 years 2 months ago
Abs 0419 SELECT-AXIS 2, UPA improved outcomes vs PBO in nr-axSpA pts across all BL inflammation subgroups; greatest benefit obs in pts with both elevated CRP and inflammation on baseline MRI. #ACR22 @RheumNow https://t.co/opaFwXgcvU https://t.co/o27ULcL8Ip

Robert B Chao, MD doctorRBC
3 years 2 months ago
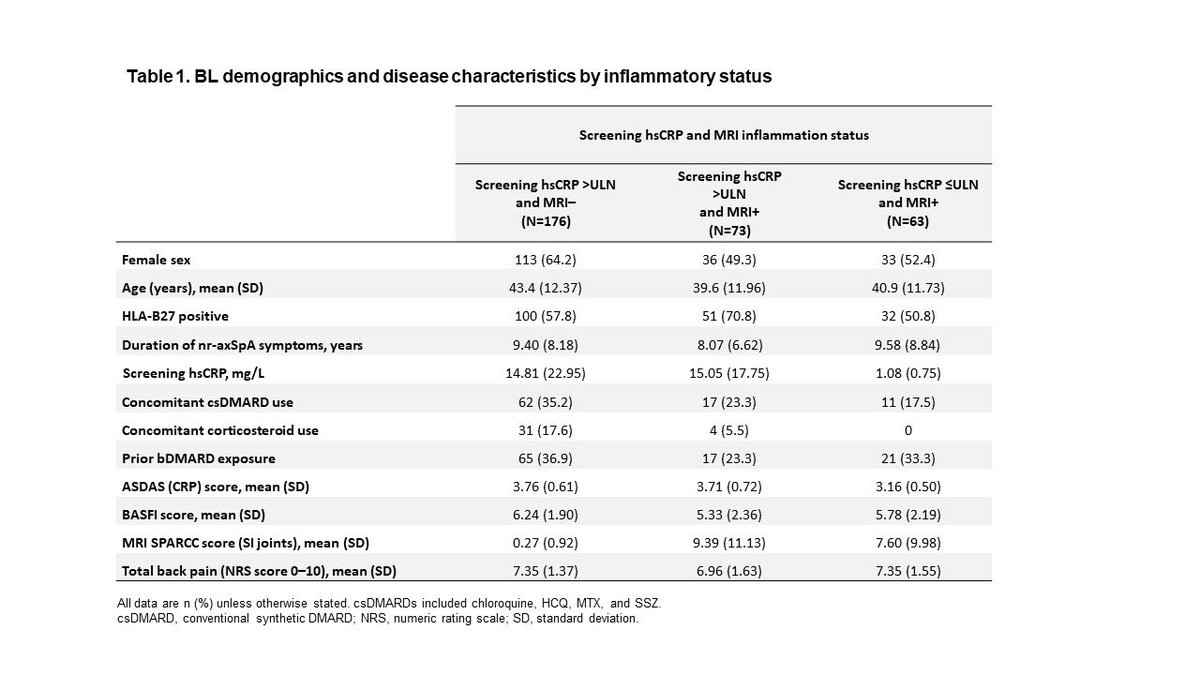
2022 ASAS-EULAR Recommendations of AxSpA management
1) NSAIDs still first line
2) Analgesics/opioids contraindicated
3) TNFi, IL-17i first line bDMARDs, followed by JAKinibs
4) Tapering but not discontinuation of bDMARDs in sustained remission
Abs#0542 @RheumNow #ACR22 https://t.co/ffaN2fMc3v

Robert B Chao, MD doctorRBC
3 years 2 months ago
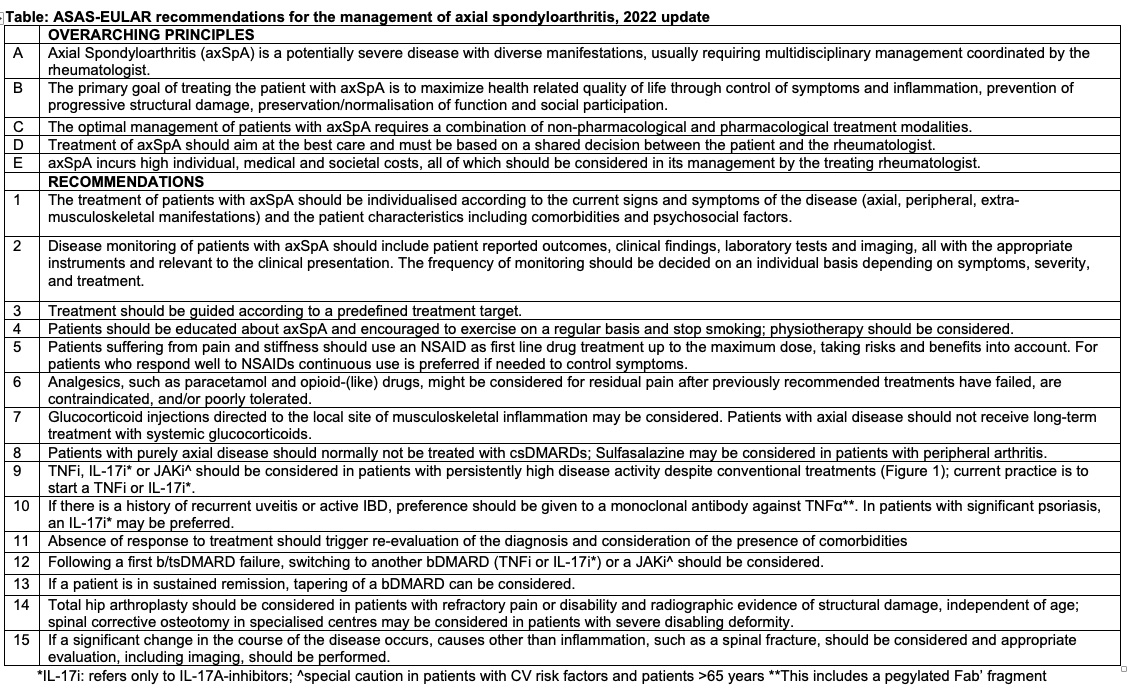
Rates of MACE and VTE with upadacitinib were infrequent and consistent with background rates in RA, PsA and AS patients. Factors associated with MACE/VTE: age>65, HTN, DM, smoking, history CV event/VTE
Abs#0510 @RheumNow #ACR22 https://t.co/AjtJF0p3QK

Dr. Antoni Chan synovialjoints
3 years 2 months ago
Key points in managing MDA5+ DM
1. Consider Tofacitinib 5-10mg bd
2. Low to mid dose Prednisolone 20-30mg tapering
3. Other Rx: Tacrolimus, IVIG
4. Poor prognostic factors: lymphopenia, raised ferritin, old age, rapid ILD progression
Wang GC, IIM session @RheumNow #ACR22 https://t.co/UF2PjAg0Mw

Dr. Antoni Chan synovialjoints
3 years 2 months ago
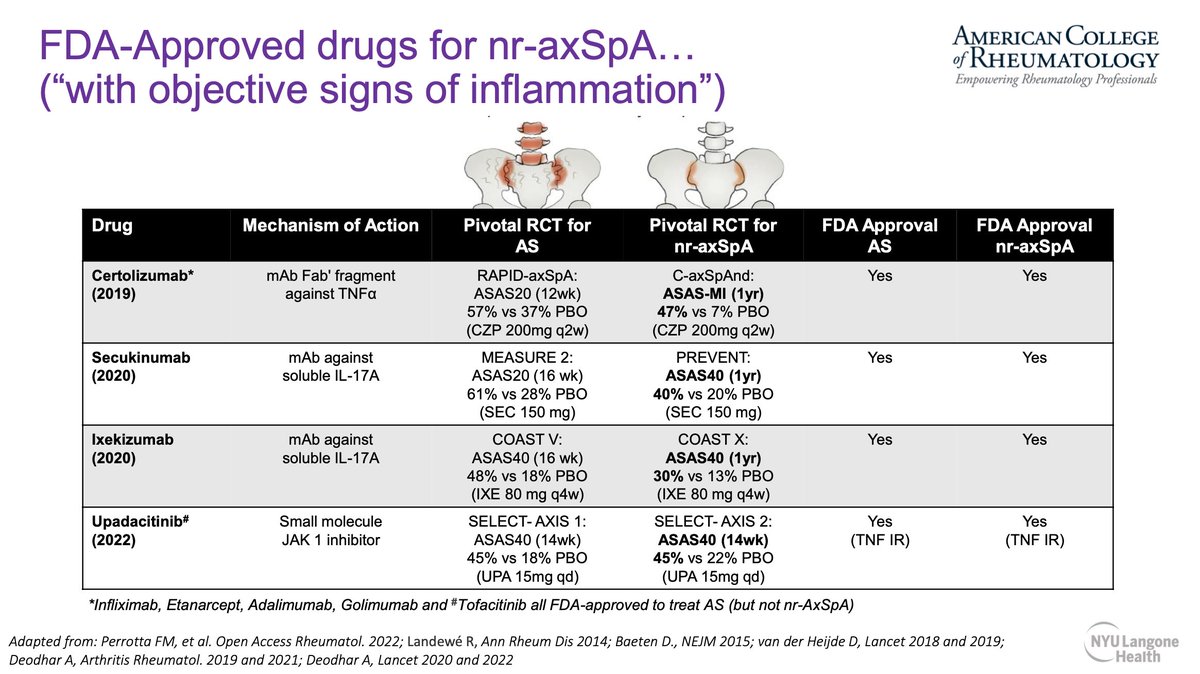
Therapies for AS and nr-AxSpA are coming together. Both AS and nr-AxSpA present with similar burden of disease. TNFi, IL-17i and JAKi now approved for use in nr-AxSpA.
Jose U Scher, SpA Review @RheumNow #ACR22 https://t.co/vcHcd7OyAU

Janet Pope Janetbirdope
3 years 2 months ago
What do you do if a person with #rheumatoidarthritis was great on 1st JAKi. It was 1st line advanced Rx @RheumNow #ACR22

Janet Pope Janetbirdope
3 years 2 months ago
OPAL data is a gem! Large Australian data studying JAKi use in #rheumatoidarthritis N=5900 Switching JAKi~JAKi-common. As in TNFi 1st line Rx had longer retention & better efficacy than 2nd & subsequent. Median 1st advanced Rx - same in all JAKs 34 MOA @RheumNow #ACR22 abst0274 https://t.co/FQU4AKXwe9

Catherine Sims, MD DrCassySims
3 years 2 months ago
Xeljanz in PsA (@pfizer funded) #ACR22 @RheumNow
⚠️safety data
-Pancytopenia
-Transaminitis
-Dyslipidemia
-Elevated CPK without #myositis
-Avoid in pregnancy and GI strictures
🤰Stop JAKi at least one month prior to conception🤰

Catherine Sims, MD DrCassySims
3 years 2 months ago
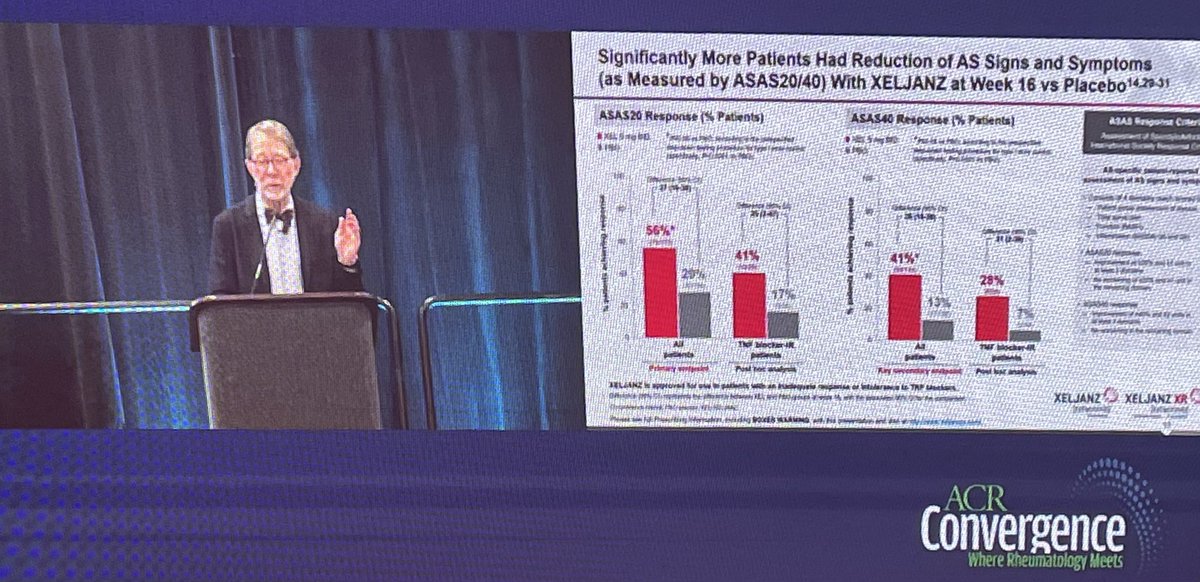
Xeljanz use in AS #ACR22 (@pfizer sponsored) @RheumNow
-Included patients with at least bilateral grade 2 SI or grade 3/4 unilateral SI
-5mg BID Xeljanz
-Significantly more patients had ASAS20/40 response, CRP reduction, & QOL, vs. placebo
-Onset of action noticed at 2 weeks https://t.co/DisKpgR0pt

David Liew drdavidliew
3 years 2 months ago
@MayoClinic data for bari in relapsing GCA caused waves at #ACR21
Spanish data looking at real-world practice: slightly longer, slower GCA, but equally encouraging outcomes
waiting for SELECT-GCA with interest! @EBRheum
ABST0464 #ACR22 @RheumNow https://t.co/pVRPXAohhc https://t.co/FgNNfPSWk3
Recently the FDA approved deucravacitinib, a highly selective TYK2 inhibitor for psoriasis. Trials are positive in psoriatic arthritis and a phase II study in SLE. What about the effects? Presentations from the ACR22 meeting may provide answers.















 Poster Hall
Poster Hall