Psoriatic arthritis

Janet Pope Janetbirdope
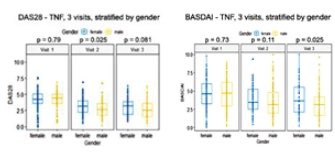

Meral K. El Ramahi, MD MeralElRamahiMD
2021 GRAPPA STRONG recs: *⃣Axial PsA/arthritis, biologic-naive or IR: TNFi, IL17i, JAKi *⃣Peripheral arthritis biologic-IR: above + IL23i *⃣Peripheral arthritis DMARD-IR: above + IL12/23i *⃣Peripheral arthritis DMARD-naive: above + PDE4i + csDMARD OP0229 #EULAR2021 @RheumNow https://t.co/Z2Nv073yoU


Robert B Chao, MD doctorRBC
Deucravacitinib (TYK2i) - phase II trial for PsA ⭐️ACR20, 50, 70 response ⭐️HAQ-DI response ⭐️LEI/SPARCC response ⭐️MDA response Abs#OP0227 #EULAR2021 @RheumNow

Robert B Chao, MD doctorRBC

Robert B Chao, MD doctorRBC
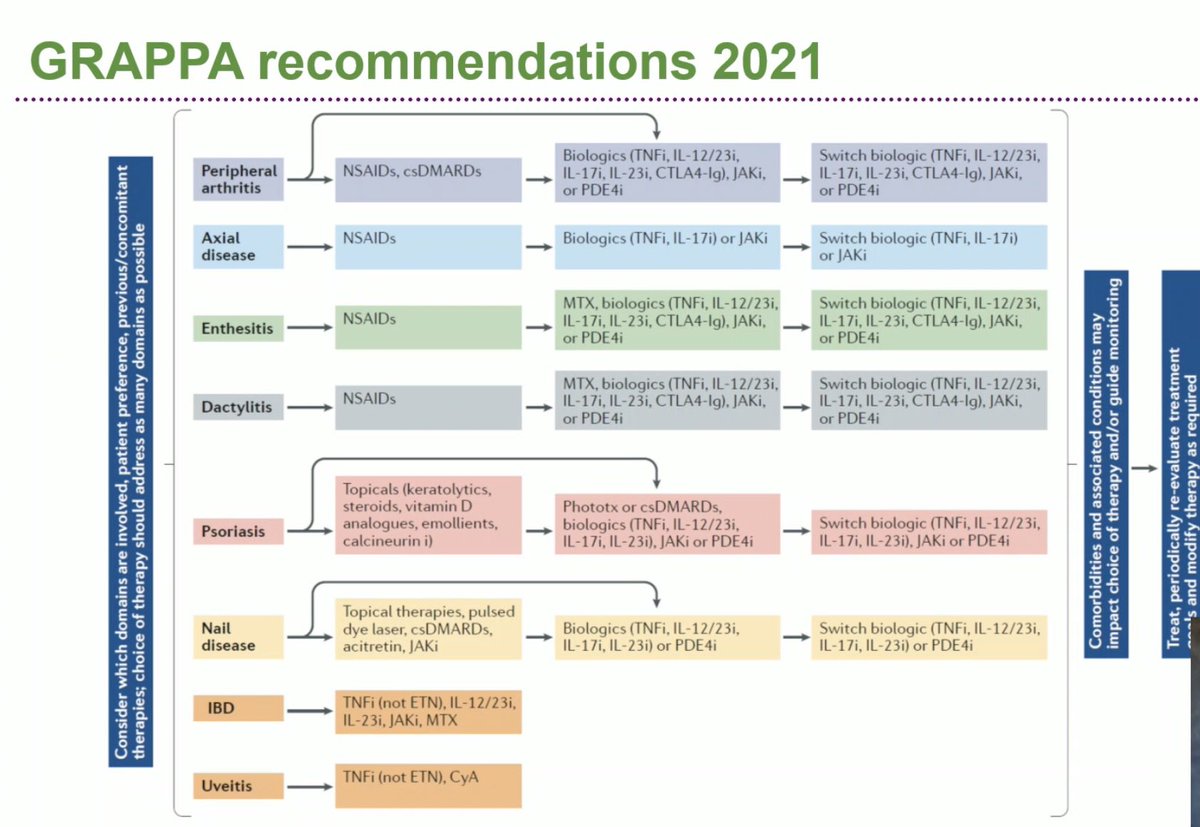

Robert B Chao, MD doctorRBC
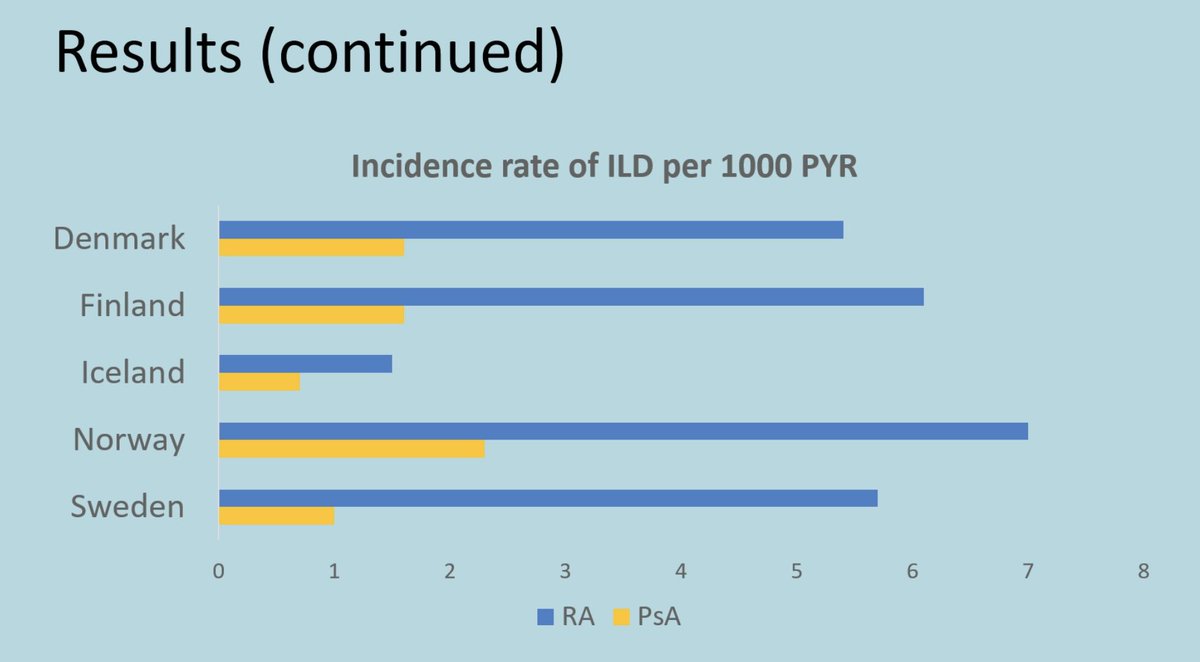

Meral K. El Ramahi, MD MeralElRamahiMD

ARD & RMD Open ARD_BMJ
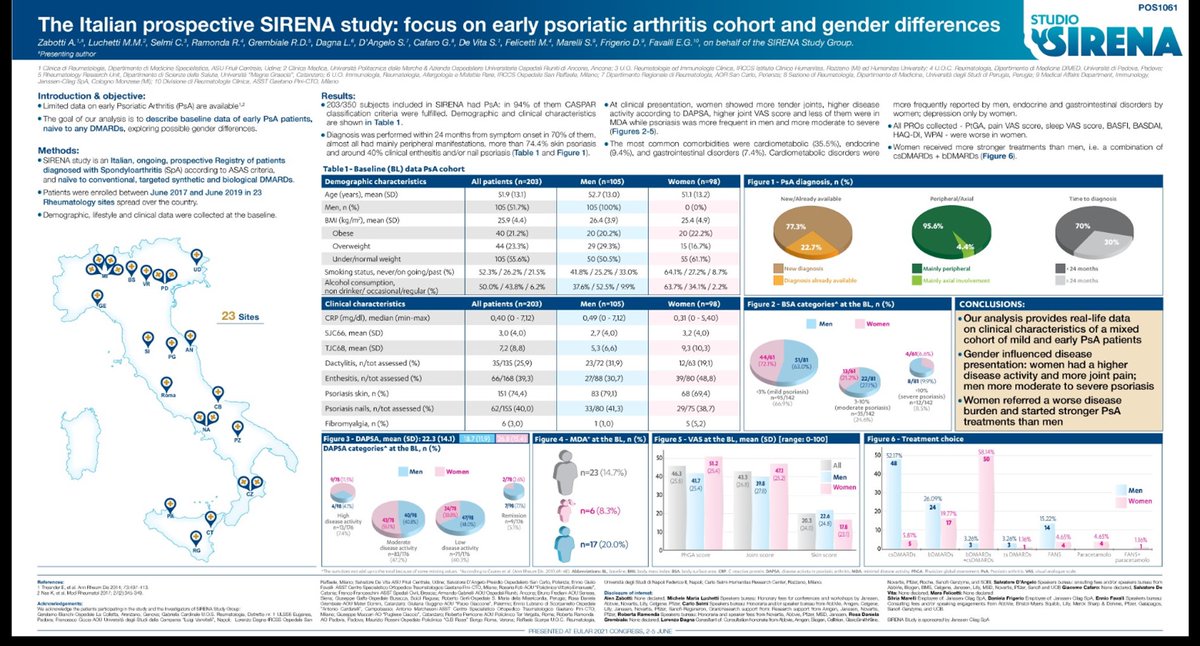

Richard Conway RichardPAConway
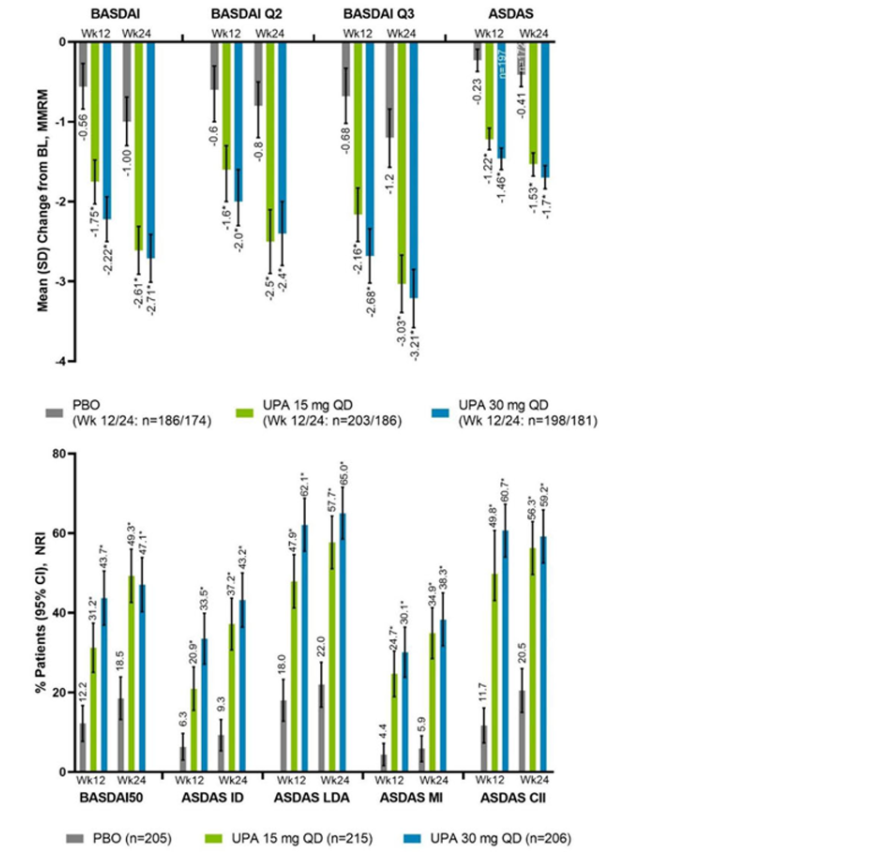

Robert B Chao, MD doctorRBC

Robert B Chao, MD doctorRBC

Robert B Chao, MD doctorRBC
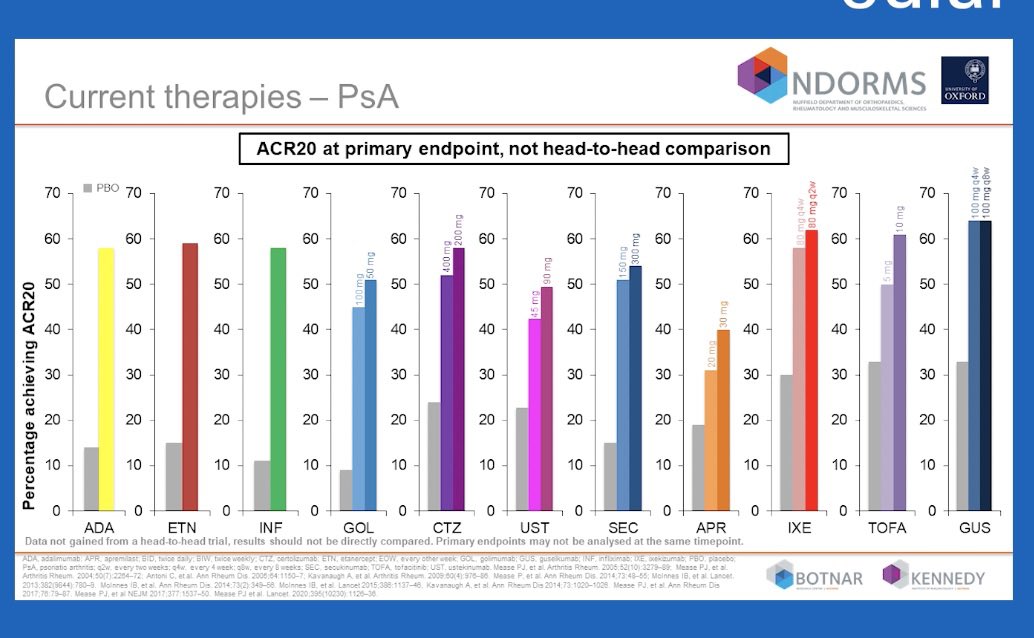

Robert B Chao, MD doctorRBC
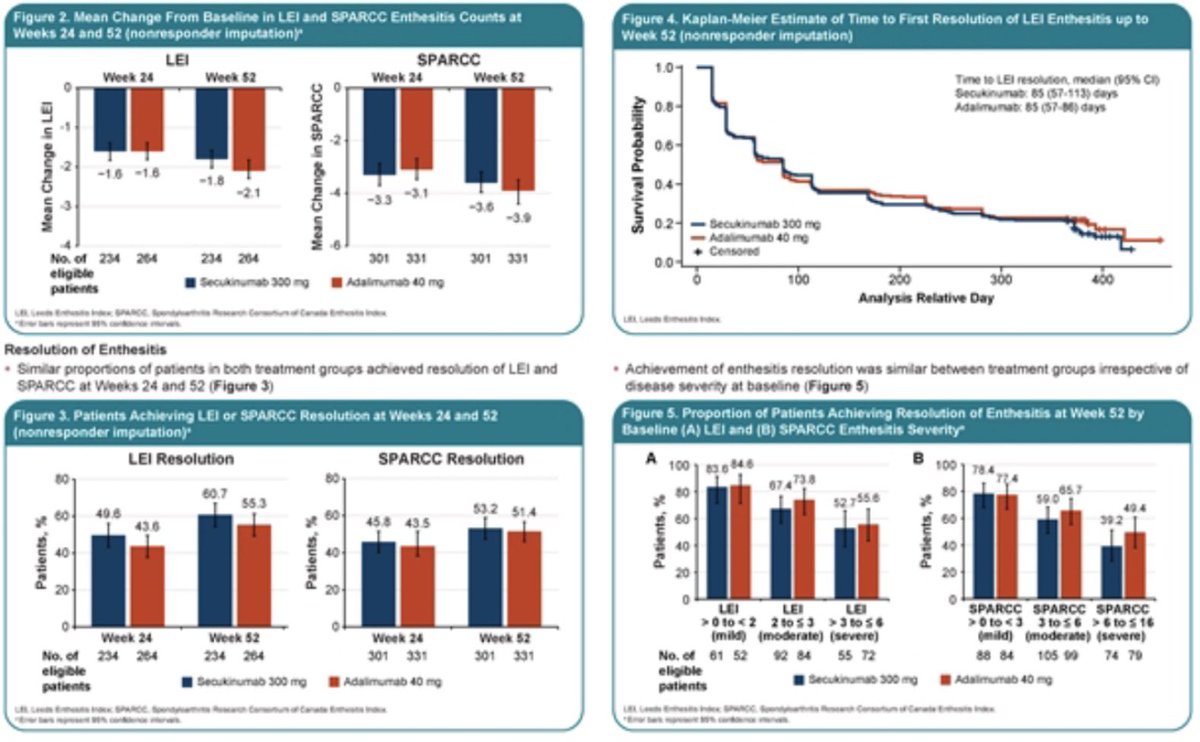

Robert B Chao, MD doctorRBC
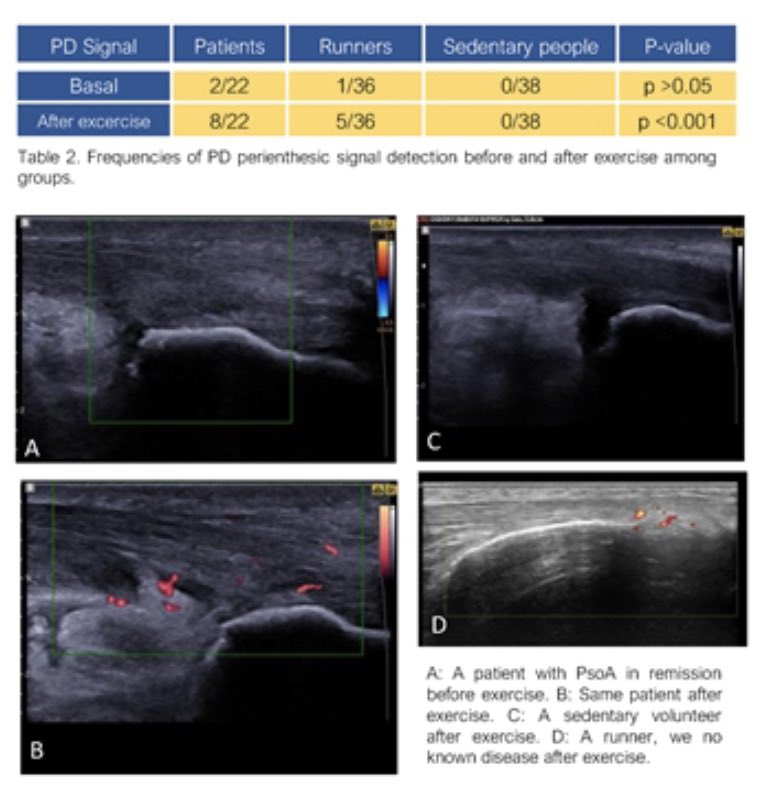








 Poster Hall
Poster Hall