Novel Rx

Mrinalini Dey DrMiniDey
8 months 3 weeks ago
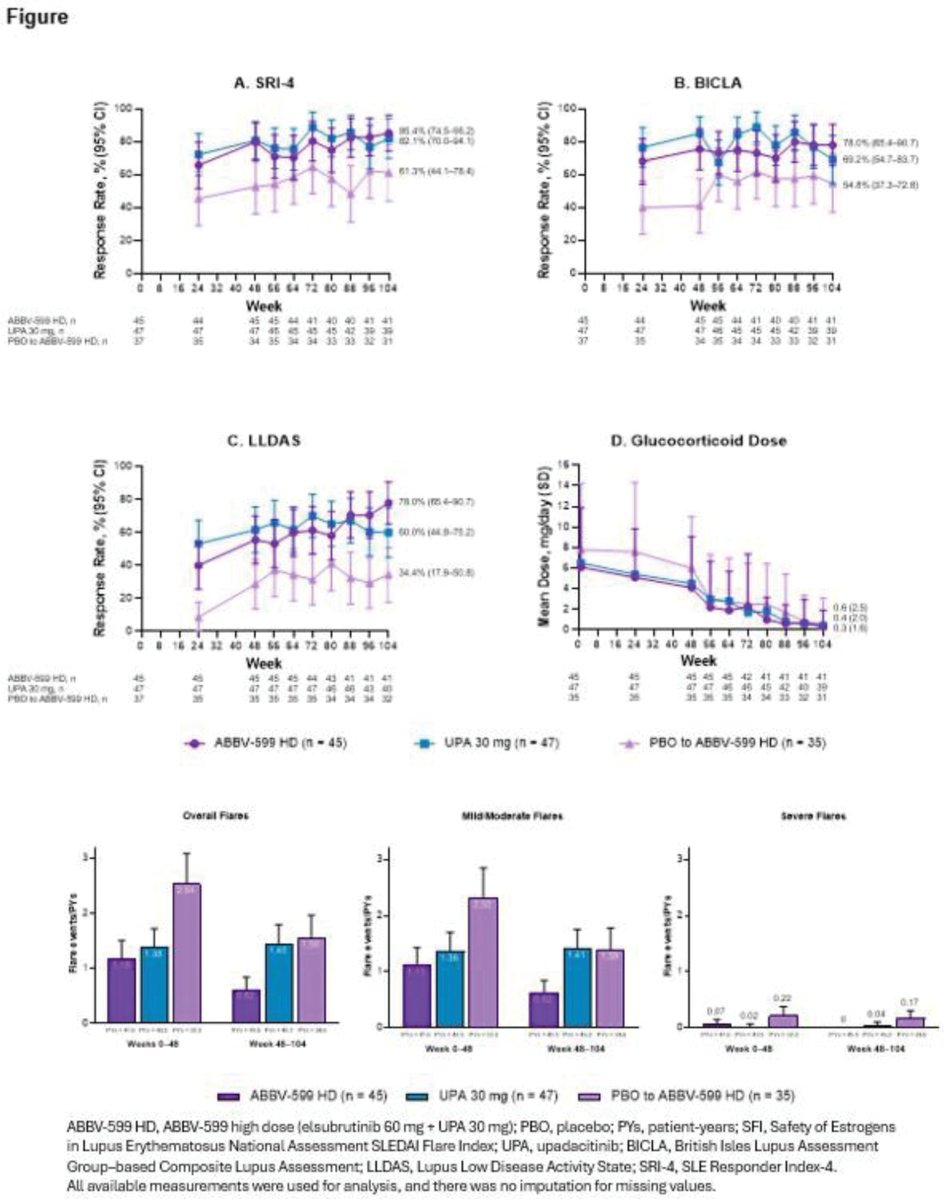
In SLEek LTE, ABBV-599 HD (BTKi+JAKi) & UPA 30mg sustained or improved disease control through 104wks: SRI-4 ≥82%, ↓flares, near steroid-free, no new safety signals. PBO-switchers improved too. Targeted oral combos look promising.
@RheumNow #EULAR2025 #OP0198 https://t.co/IHXC2iD0h6

Dr. John Cush RheumNow
8 months 3 weeks ago
Secukinumab Use in Refractory Giant Cell Arteritis
In 2023, the phase 2 TitAIN study showed that the effectiveness and safety of secukinumab in 52 patients with giant cell arteritis (GCA) who had an inadequate response to tocilizumab. While we await the results of a larger https://t.co/pG3aN3o4GZ

Janet Pope Janetbirdope
8 months 3 weeks ago
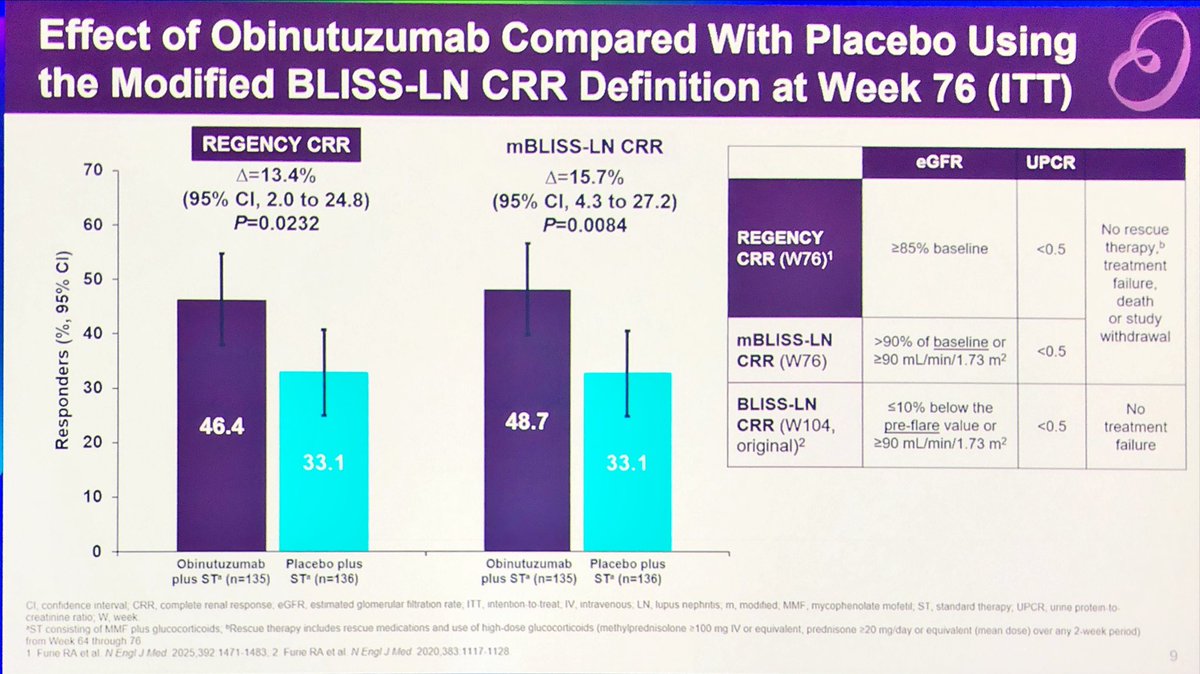
#Oninutuzumab works in
#lupus #nephritis #LN
no matter how you vary the
#renal #responses
Of various definitions of
#Complete & #Partial #renal #responses
abst#OP0006
#EULAR25 @eular_org @RheumNow
Not surprised 😲 https://t.co/Zn2lXkddez

Janet Pope Janetbirdope
8 months 3 weeks ago
1 shot will do!
#symptomatic #Knee #OA given #gene #therapy #intra-#articular
Seemed to last up to 104 weeks!
But no placebo
small safety study but sustained #WOMAC responses
Needs large #RCT but v interesting
#EULAR2025 @RheumNow @eular_org
Abst# POS0492 https://t.co/hqTqBorrbA


Adela Castro AdelaCastro222
8 months 3 weeks ago
Secukinumab for PMR?
-post hoc analysis of the TitAIN study (phase 2 RCT on new onset/relapsing GCA) showed:
-Numerical reduction in patients experiencing PMR symptoms when treated with secukinumab compared to placebo.
-Safety profile was similar to the overall GCA study

Antoni Chan MD (Prof) synovialjoints
8 months 3 weeks ago
In vitro, balinatunfib (TNFR1-selective inhibitor) preserved Treg expansion in CD4+ T cells co-cultured with IL-2 and memTNF (Treg 8.99 percent, p<0.0001), unlike adalimumab and etanercept which reduced Tregs by 27.5 to 41 percent. Confirms TNFR2 sparing with selective TNFR1 https://t.co/xaczz87Xrd


Antoni Chan MD (Prof) synovialjoints
8 months 3 weeks ago
VEGF-Grab (PB101/PB102), dual VEGF/PlGF decoy receptor, inhibited angiogenesis, RA-FLS invasion, and Th17 cell differentiation in RA and MS models. PB102 reduced IL-17 and GM-CSF co-expressing Th17 cells, suppressed pannus and joint destruction in CIA, and outperformed IFN-β in https://t.co/qhVRYT4RbK


Md Yuzaiful Md Yusof Yuz6Yusof
8 months 3 weeks ago
#EULAR2025 Abstr#OP0002 Promising new mode of action therapy for #myositis. Phase 2 RCT of efgartigimod (FcRn-i; coformulated with recombinant PH20) showed improvement in TIS & other key endpoints vs PBO at Wk24. Injection reaction common (23%). Will proceed to Phase 3 @RheumNow https://t.co/SuMolfcDkS


Janet Pope Janetbirdope
8 months 3 weeks ago
Cool technology reduces dose interval of #IL17AFi
To…every 6 to 12 months!
#Antibody ORKA-002 has end of arms w YTE substitution prolonging circulation of drug
Lasts longer than #bimekizumab
Easier for adherence!
#EULAR2025 @RheumNow @eular_org #abstPOS0016 https://t.co/KcKaIe0WMs

Despite the advances in the treatment of PsA with biologic (bDMARD) and targeted synthetic (tsDMARD), less than half of patients with this condition achieved remission or low disease activity. Combination DMARD treatment is often used in order to achieve remission or minimal disease activity. The standard practice is to use a conventional synthetic (csDMARD) with a bDMARD. The use of the combination of bDMARD with a tsDMARD such as a JAKi or

Nelly ZIADE 🍀 Nellziade
8 months 3 weeks ago
❓️How effective and safe is it to combine bDMARDs and tsDMARDS (i.e. TNFi/ IL17i + JAKi/TYKi) in #psoriatic_arthritis?
🅰️ Reassuring data from a case-series study presented by Andre Lucas Ribeiro
#EULAR2025
OP0090
@RheumNow
#Strategy https://t.co/W8fuzSgxbJ


Antoni Chan MD (Prof) synovialjoints
8 months 3 weeks ago
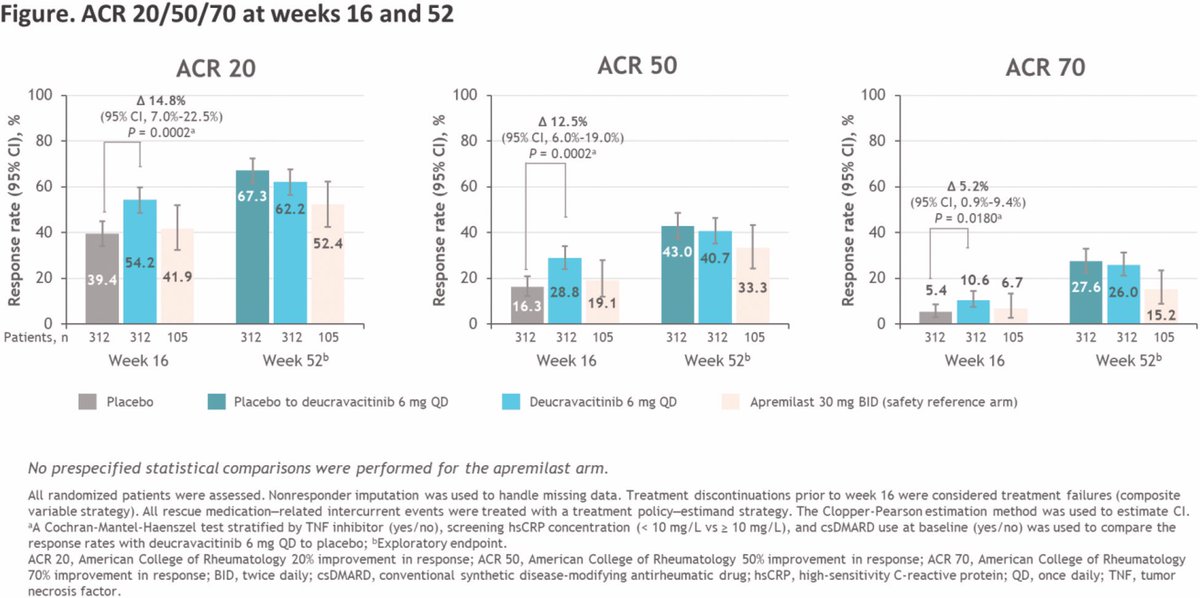
Efficacy and safety of deucravacitinib up to week 52 in the POETYK PsA-2 study
•ACR20 at W16: 54.2% (deucravacitinib) vs 39.4% (placebo), p=0.0002
•PASI75: 40.9% vs 15.4%, p<0.0001
•MDA: 25.6% vs 14.7%, p=0.0007
•FACIT-F: +2.5 vs +1.8
•SAE: 1.9% (low)
TYK2 inhibition shows https://t.co/JXTZRk2itA

Jiha Lee JihaRheum
8 months 3 weeks ago
In propensity-matched RA cohort (n=2,449 each) on JAK inhibitors, adding a GLP-1 agonist reduced risk of: • Acute coronary syndromes (RR 0.65, p=0.0009) • DVT (RR 0.69, p=0.007) Overall CV events ↓ by 33% (RR 0.67, p=0.0007)
#EULAR2025 @RheumNow
Abstract#OP0069 https://t.co/jWI6tvri8O


Adela Castro AdelaCastro222
8 months 3 weeks ago
Xeligekimab in AS:
-IL-17A blocker
-Phase 3 study on Chinese pts showed sustained efficacy until week 48.
-Significant improvement on DAI measures as well.
-Fully humanized IgG4 mab may last longer in system and possible less ADAs.
Abstract #OP0102 #EULAR2025 @RheumNow https://t.co/vyNHZ9ssFt









 Poster Hall
Poster Hall