IL-17

Meral K. El Ramahi, MD MeralElRamahiMD
4 years 7 months ago
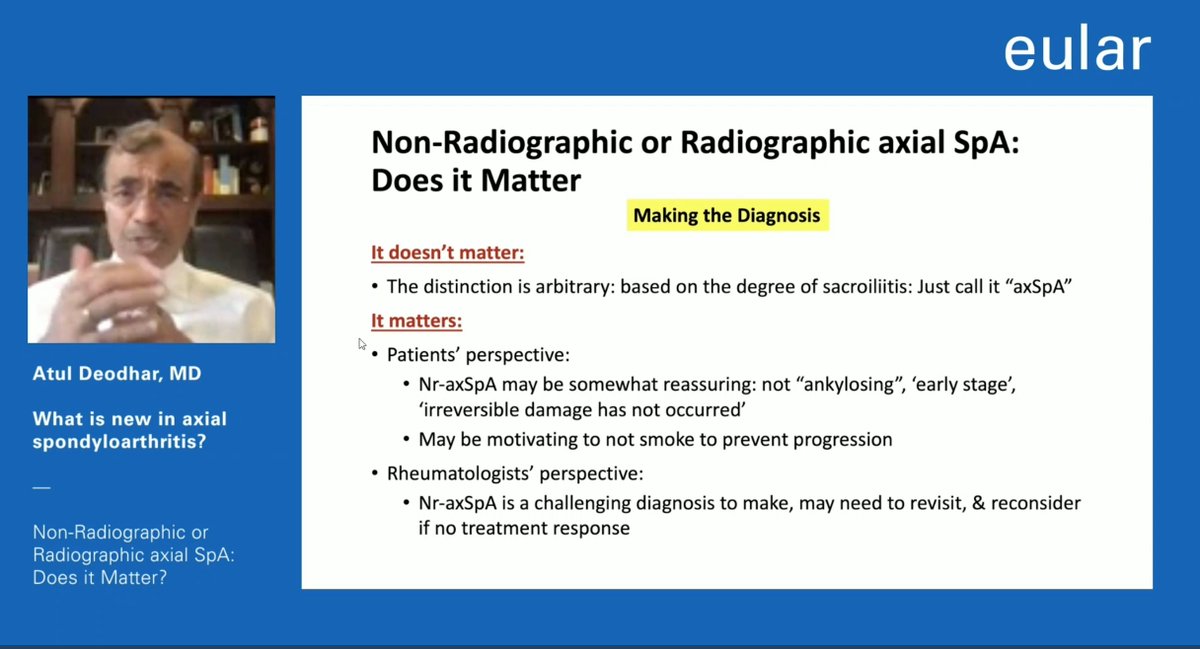
#EULAR2021 bDMARD and TNF-i naive patients w/ r-axSpA without evidence of objective inflammation (normal CRP & MRI ) showed ASA40 response w/ ixekizumab @RheumNow https://t.co/5KT8A81auq

Dr. John Cush RheumNow
4 years 7 months ago
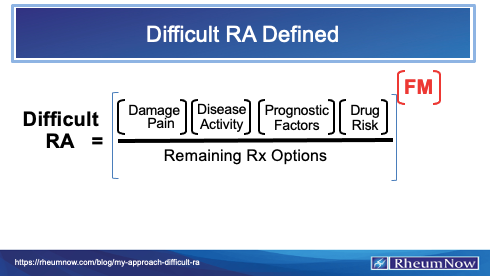
New download available!
Difficult RA Defined
https://t.co/6WGMEiwiUk https://t.co/VTCm4cuyRe







 Poster Hall
Poster Hall