IL-17

2 years 11 months ago
IL-17i expressed in overuse tendinopathy. Secukinumab for tx of rotator cuff tendinopathy?
⭐️Phase II study - no difference in rotator cuff score (WORC) vs placebo
⭐️no difference in pain score
Abs#POS0022
#EULAR2021 @RheumNow

2 years 11 months ago
➡️ASTERA = PIII trial that showed superior efficacy of Netakimab (IL-17Ai) vs PBO in pts w/ active r-axSpA. Excluded pts prev tx w/ 2 or more TNFi
➡️OP0142 = sub-analysis showing Netak ⬇️ dz activity in AS pts irrespective of sacroillitis on MRI at b/l
#EULAR2021 @Rheumnow https://t.co/faT3M0xQiR


2 years 11 months ago
I haven’t used it but… Netakimab (IL-17i) approved for treatment of PsO, AS, and PsA in Russia and Belarus
⭐️⬇️AS activity w/ or w/o MRI sacroilitis
Abs#OP0142
#EULAR2021 @RheumNow

2 years 11 months ago
⭐️All cancer excluding NMSC - 0.9HR TNFi vs cDMARD
⭐️Squamous cell CA - 1.2HR of TNFi vs cDMARD
⭐️No overall increase in all CA: RTX, TOCI
⭐️Abatacept 1.21HR for all cancer, mainly NMSC
⭐️needs more data on JAKs, IL-17, IL-23
Abs#6916
#EULAR2021 @RheumNow

2 years 11 months ago
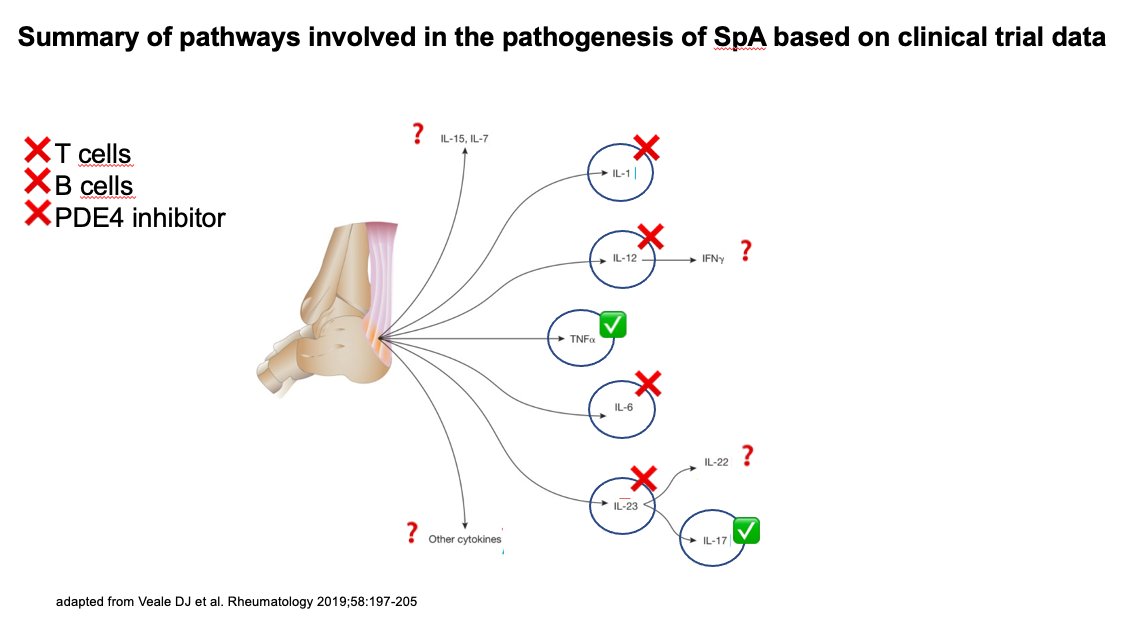
From bedside to bench–unexpected results of clinical studies that changed our understanding of the disease #EULAR2021.
“SpA” talk by Prof. Désirée van der Heijde.
Inhibition of TNF, IL-17, IL-23, IL-6, IL-1, co-stimulation and B cell depletion in immunology perspective 👏
/TK https://t.co/JFr3VC8sXY

2 years 11 months ago
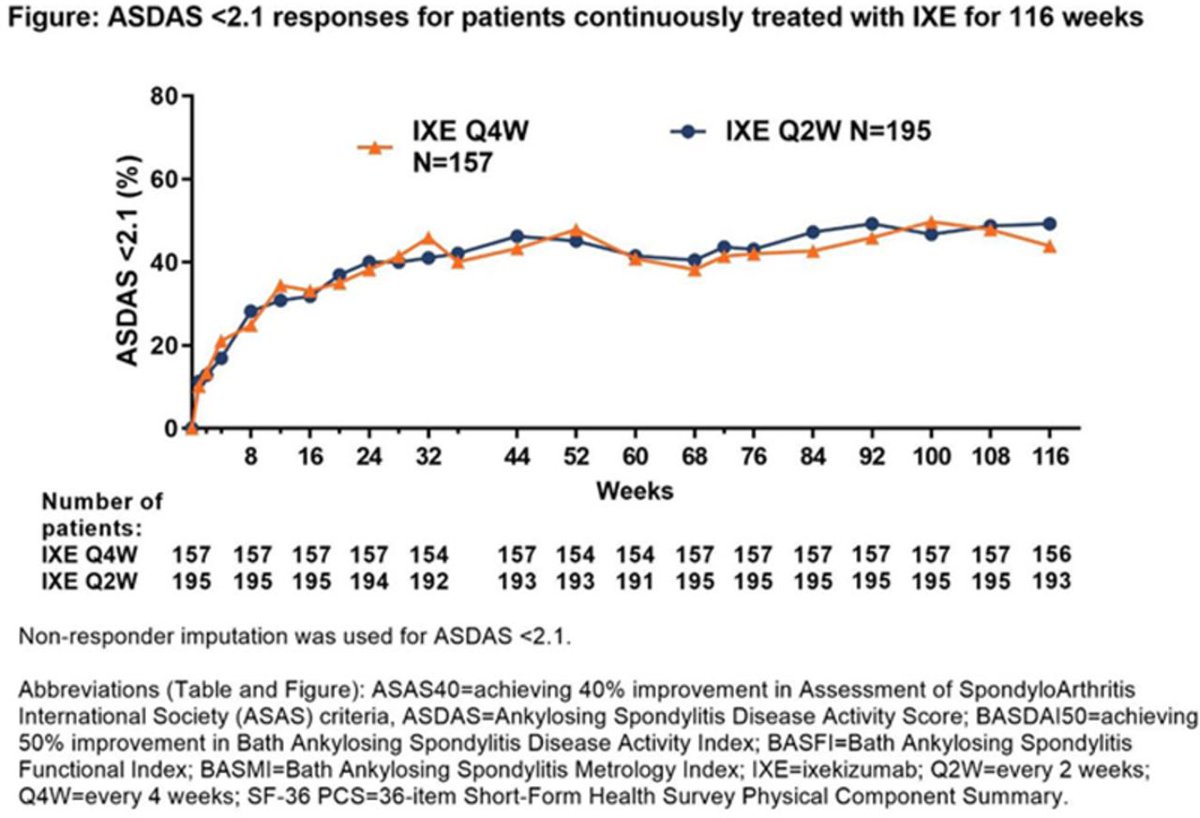
COAST-Y 2 yr results: In r- and nr-axSpA, IXE tx led to consistent & sustained long-term improvements in disease activity & QoL. No new safety signals. #EULAR2021 poster #POS0912 @RheumNow https://t.co/W2YoyA4K14 https://t.co/lv0GLU2WTF

2 years 11 months ago
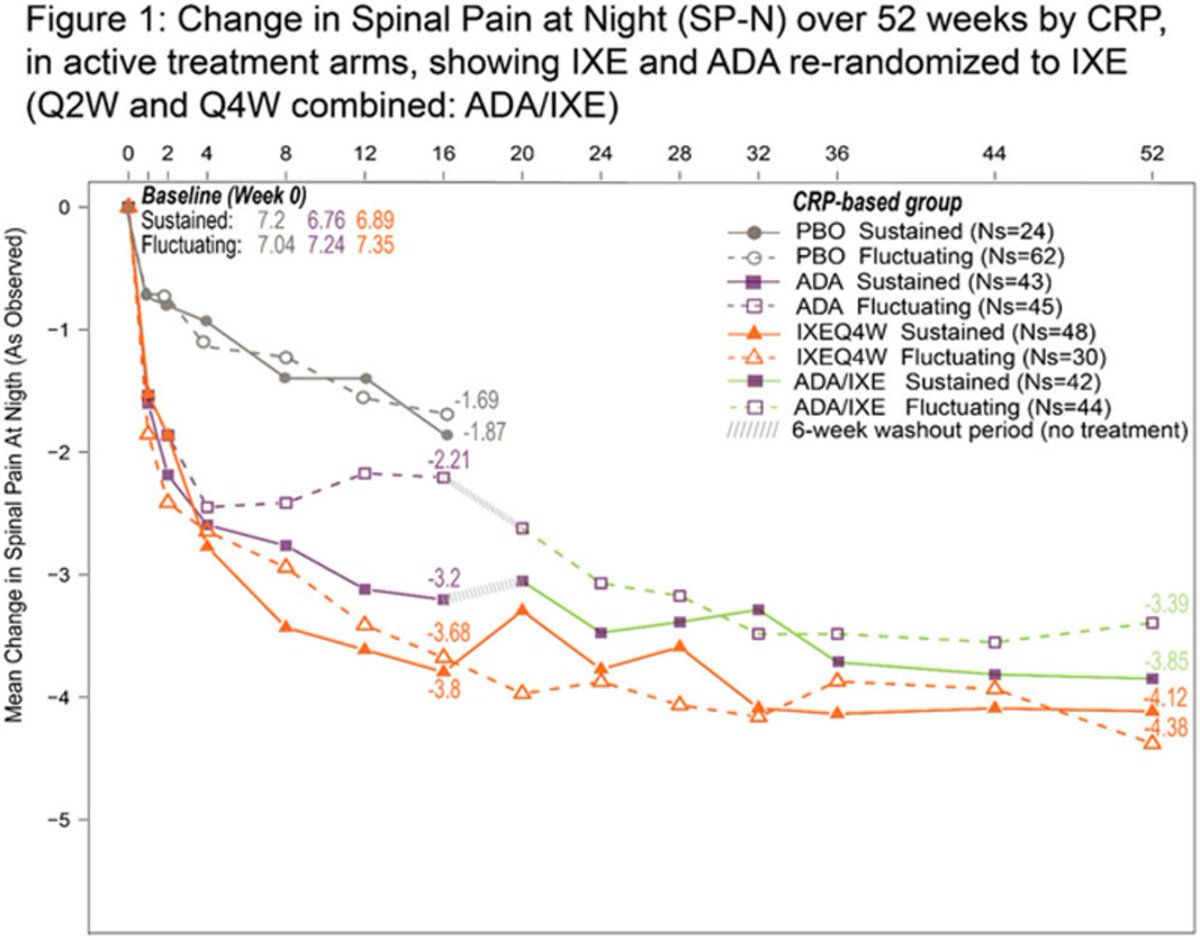
COAST-V: IXE ⬇️SP-N & spinal pain in r-axSpA pts w/ or w/o ⬆️ CRP or morning stiffness. Pts treated w/ ADA re-randomized to IXE showed further⬇️in SP-N & spinal pain. Poster #POS0901 #EULAR2021 @RheumNow https://t.co/46qiqrohbX https://t.co/uB15oTwztV

2 years 11 months ago
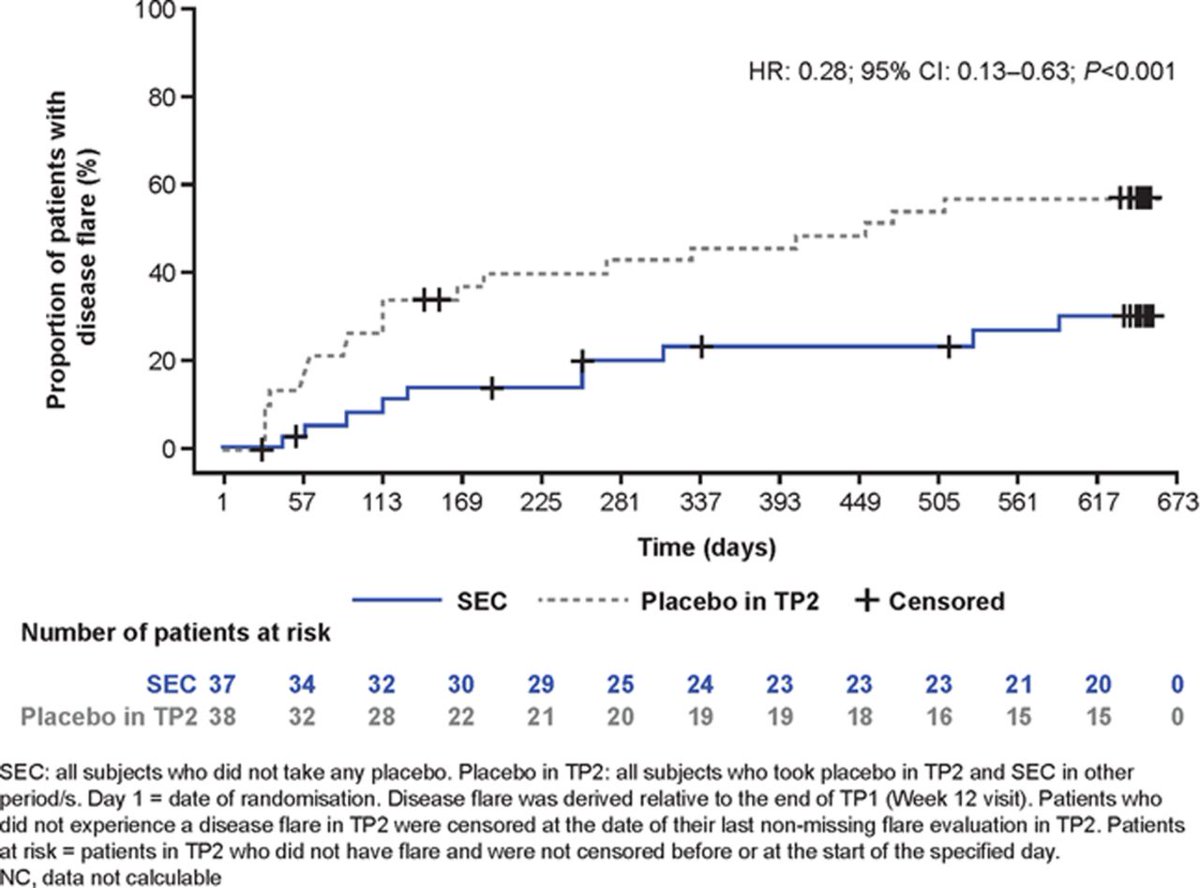
ERA & JPsA children & adolescents tx'ed w/ SEC, JUNIPERA 104wk data: longer time to flare vs PBO. NRI analysis: 87.2%, 83.7%, 67.4%, 38.4% and 24.4% achieved JIA ACR 30/50/70/90/100, respectively. No new safety signals. #EULAR2021 @RheumNow Abstract #LB004 https://t.co/reWawBWfqh https://t.co/9gzKKU1xrm












 Poster Hall
Poster Hall