Psoriatic arthritis

Dr. Antoni Chan synovialjoints
1 year 6 months ago
Synovial tissue specimens were processed for H&E-based semiquantitative synovitis quantification (KSS) in biopsies in PsA. KSS was significantly higher in cs/b-DMARDs resistant patients than naive PsA(p<0.0001) and PsA in remission/LDA (p<0.0001). KSS directly correlated with… https://t.co/cHIw8zRByK https://t.co/vppmG7JwC8
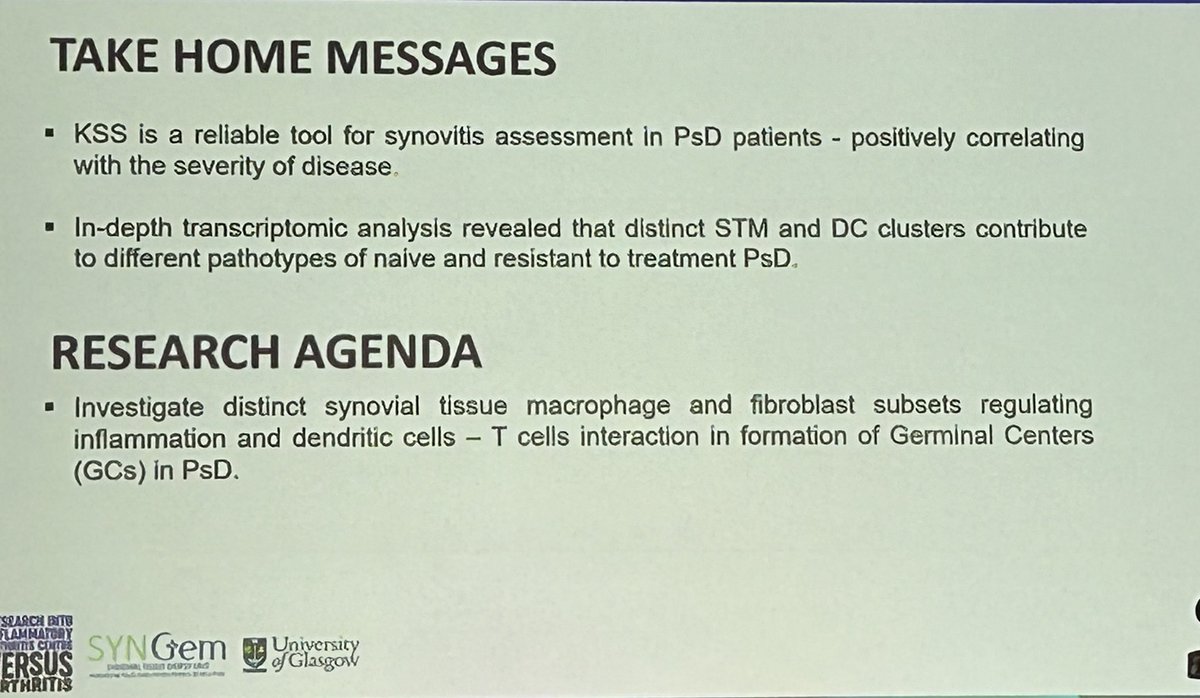

Dr. Antoni Chan synovialjoints
1 year 6 months ago
In a Swedish-Danish cohort study on patients with PsA, treatment with TNFi was associated with a small increased risk of NMSC and with non-significant numeric risk increases for both BCC and SCC compared with bDMARD naïve #EULAR2024 Abstract 0150 @RheumNow https://t.co/vC4pgSKmfY


Dr. Antoni Chan synovialjoints
1 year 6 months ago
Can we prevent psoriatic arthritis? Highlights from #EULAR2024 that help us understand this topic better @RheumNow https://t.co/E8Wy8FH4oU
Wednesday was Day One at EULAR 2024 in Vienna. While the day was a slow start, the poster halls and auditoriums quickly filled with thousands of rheumatologists, eager to reunite at this international educational forum. Below are a few of my favorites from Day 1.
Psoriatic arthritis (PsA) can affect up to 30% of patients with psoriasis (PsO). Understanding the factors that predispose patients with PsO who progress to PsA is the first step in understanding how early interventions in PsO may augment the progression to PsA. At #EULAR2024, there are a few presentations on this topic that will help us understand this progression from PsO to PsA better.

Aurelie Najm AurelieRheumo
1 year 6 months ago
200 millions pts PSO
1st line, Reduction risk PsA: IL 12-23 37% & IL-23 39% vs. TNFi
2nd line ttmt, IL12-23i 32% & IL-23i 31%
IL-23i 47% lower proba PsA than IL-17i 3 & 5 yrs
Can't exclude some channeling bias, but that's a big signal!
@RheumNow OP0010 #EULAR2024 #EULARBest

Dr. Antoni Chan synovialjoints
1 year 6 months ago
A potentially notable association between TNFi in PsA and an increased risk of ischemic heart diseases and heart failure compared to IL-17i treatment. Limitations of the study noted. This may be due to patient selection and channeling of patients to the TNFi group and more… https://t.co/Q2LotHZwjQ https://t.co/uwl6vBIMsb
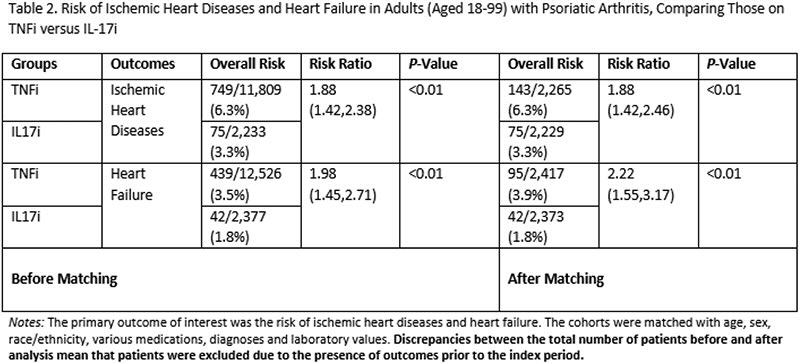

Mrinalini Dey DrMiniDey
1 year 6 months ago
💊 Difficult-to-treat #SpA & #PsA
🤔 What are the challenges in assessing persistently active disease?
🗣️ Vinod Chandran takes us through the pearls and pitfalls in assessing D2T disease
🌎 Great to see #comorbidities highlighted
@RheumNow
#EULAR2024 https://t.co/x4jUTOC3li
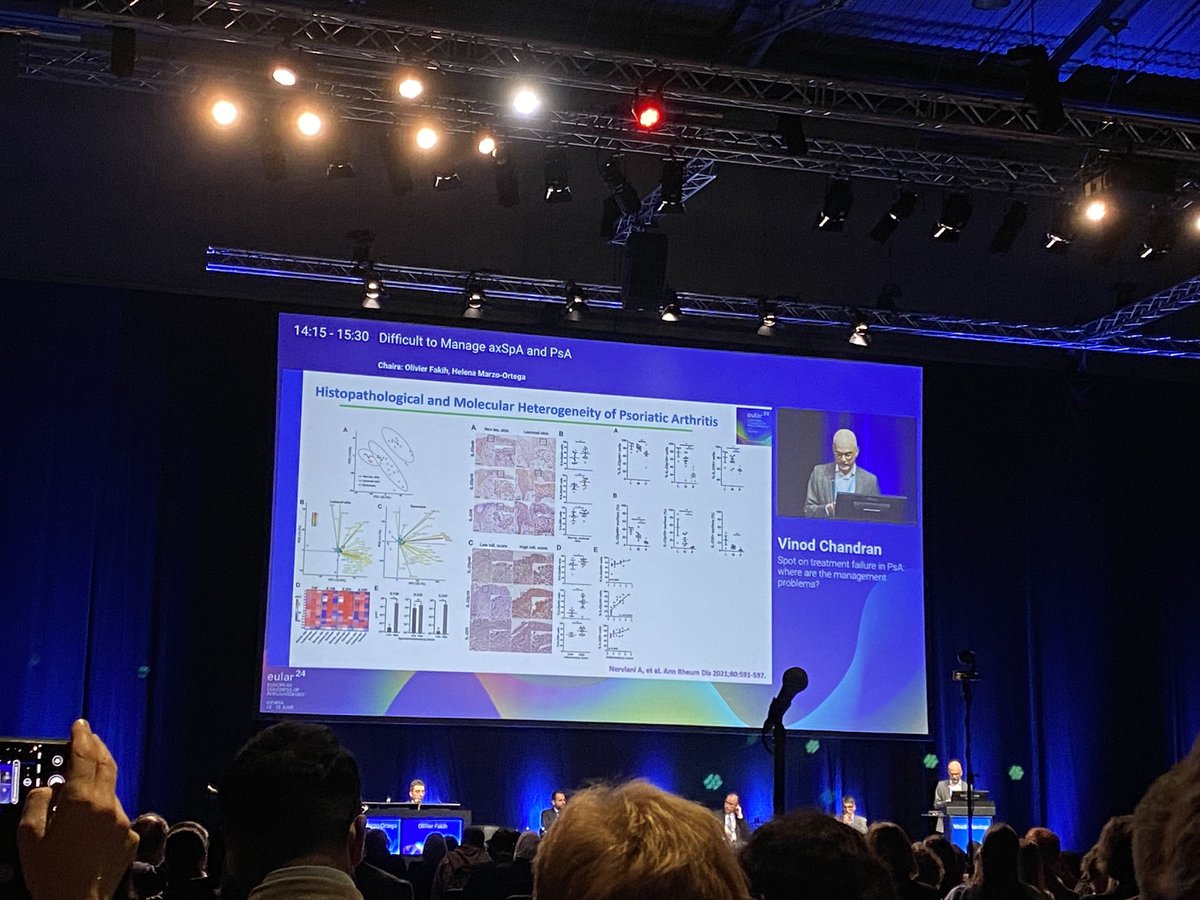

Dr. Antoni Chan synovialjoints
1 year 6 months ago
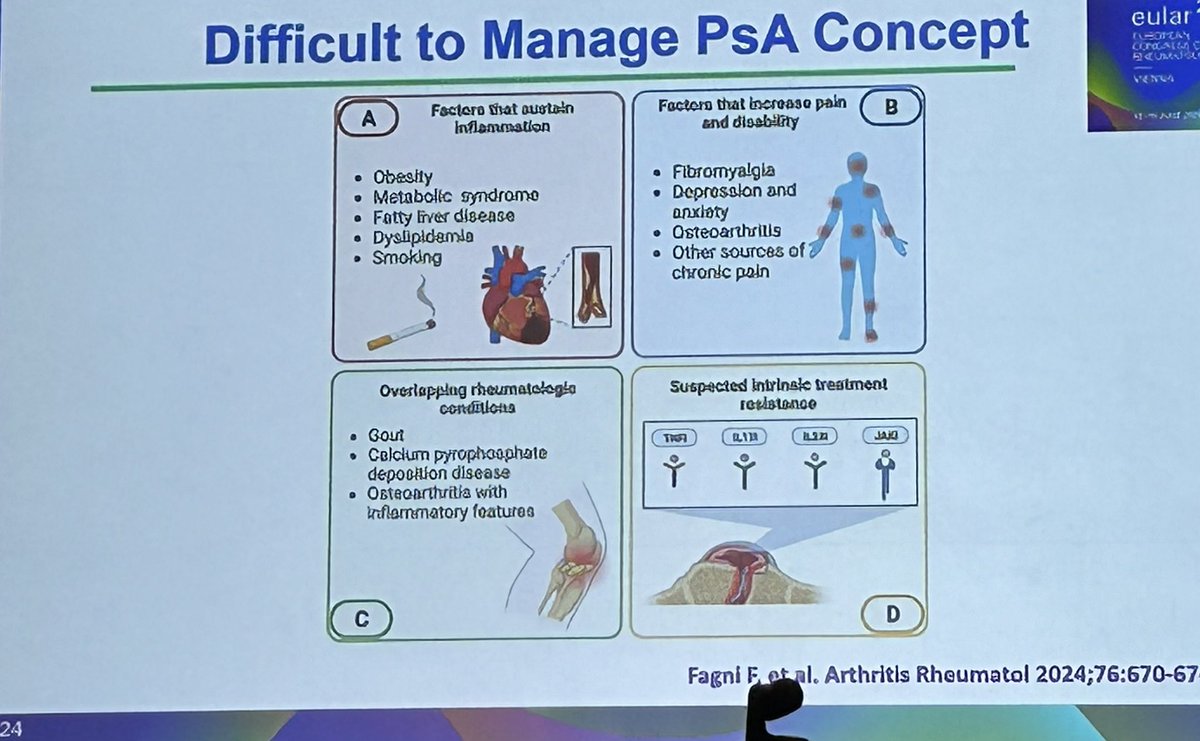
Areas to consider in difficult to manage PsA and research agenda #EULAR2024 Chandran V @RheumNow https://t.co/sp4tkAivLa


Dr. Antoni Chan synovialjoints
1 year 6 months ago
Significant proportion of patients in PsA do not achieve remission across the domains. This can be due to disease heterogeneity, domain differences, comorbidities. There is a need to study the use of combination ts and bDMARDs. Difficult to Manage PsA session #EULAR2024 Ribeiro,… https://t.co/Wt3jgtIR9N https://t.co/VzCApikqek

Peter Nash drpnash
1 year 6 months ago
Grappa - distinguish D2T from complex to manage - objective signs of inflammation imaging - US or MRI not lab markers @rheumnow #EULAR2024

Peter Nash drpnash
1 year 6 months ago
almost half PsA patients have 3 pr more co-morbidities and 67% of those surveyed do not think rheumos should not manage them - when did we stop being physicians ? @rheumnow #EULAR2024

Peter Nash drpnash
1 year 6 months ago
« difficult to treat PsA » how mich combination obesity, non-inflammatory MSK pain, fibromyalgia and depression making assessment tricky and risk of rattling through therapies fast! @rheumnow #EULAR2024















 Poster Hall
Poster Hall