Drug Safety

Mrinalini Dey DrMiniDey
8 months 3 weeks ago
#MITIGATE subgroup analysis: Inebilizumab reduced flares & boosted remission across all #IgG4-RD subgroups. HR for flare vs PBO: 0.12-0.16 across sex/race. Similar results for all subgroups in achieving flare-free, treatment-free complete remission.
@RheumNow #EULAR2025 #OP0189
Two recent studies suggest there is no significant benefit of early biologics over standard step-up care with methotrexate2,3, but these did not select for poor prognosis.

Aurelie Najm AurelieRheumo
8 months 3 weeks ago
There is still hope for vagal nerve stimulation in RA!
RCT RESET-RA 240+pts multibioIR
Implantable cervical device
Meets Primary endpoint ACR 20 wk12
Subgroup 1 prior bioDMARD ACR 20 wk12 46% vs 19% sham
EULAR Good response 61% vs 42% sham Wk 12
SAE rate 1.7%
#OP0190 #EULAR2025 https://t.co/DCON01XXgd


Mrinalini Dey DrMiniDey
8 months 3 weeks ago
2-year #MANDARA data: Benralizumab shows durable remission, eosinophil suppression & OGC-sparing in EGPA. ~62-68% in remission at wk104. Low relapse rate, no loss of asthma control or decline of lung function. No new safety signals.
@RheumNow #EULAR2025 #OP0166 https://t.co/kXh2q2ilJF


Mrinalini Dey DrMiniDey
8 months 3 weeks ago
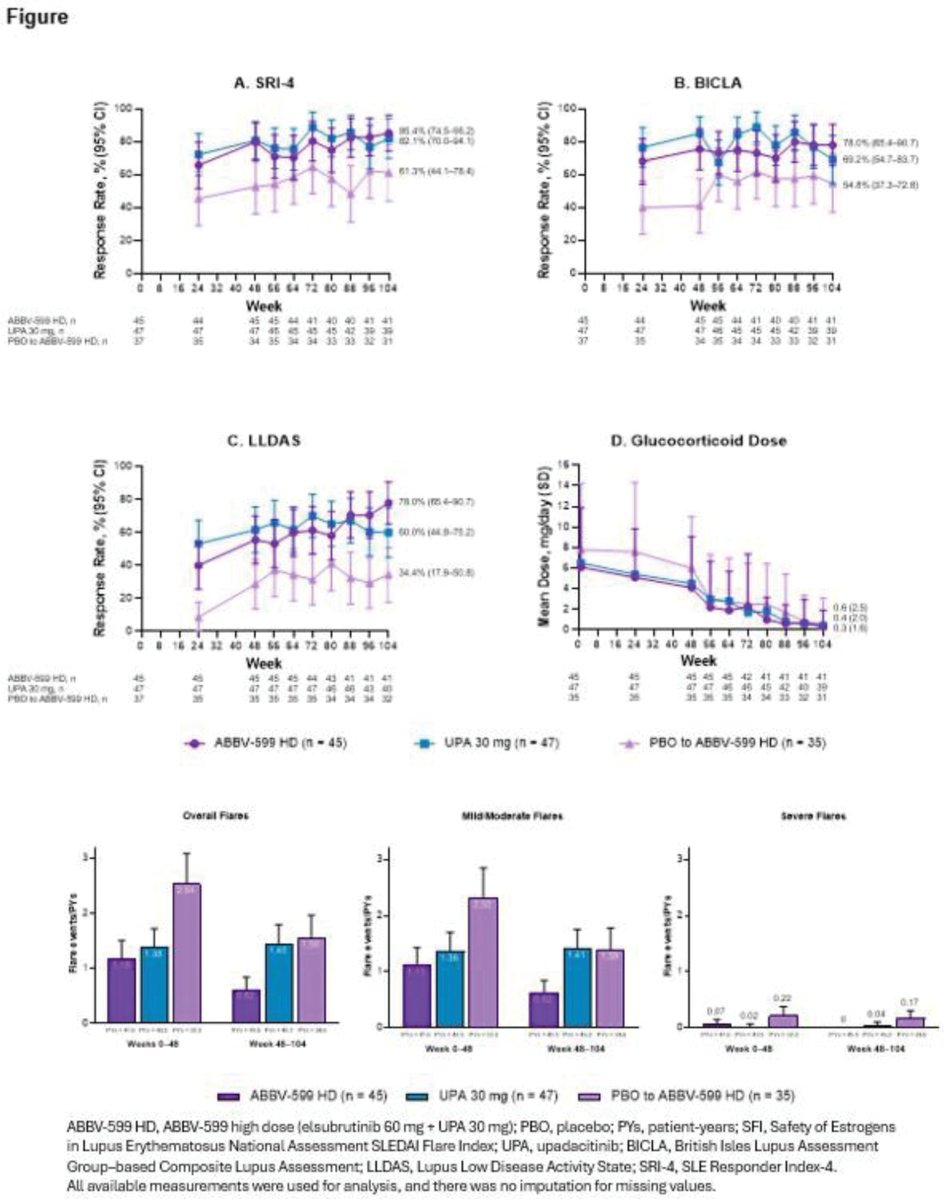
In SLEek LTE, ABBV-599 HD (BTKi+JAKi) & UPA 30mg sustained or improved disease control through 104wks: SRI-4 ≥82%, ↓flares, near steroid-free, no new safety signals. PBO-switchers improved too. Targeted oral combos look promising.
@RheumNow #EULAR2025 #OP0198 https://t.co/IHXC2iD0h6

Md Yuzaiful Md Yusof Yuz6Yusof
8 months 3 weeks ago
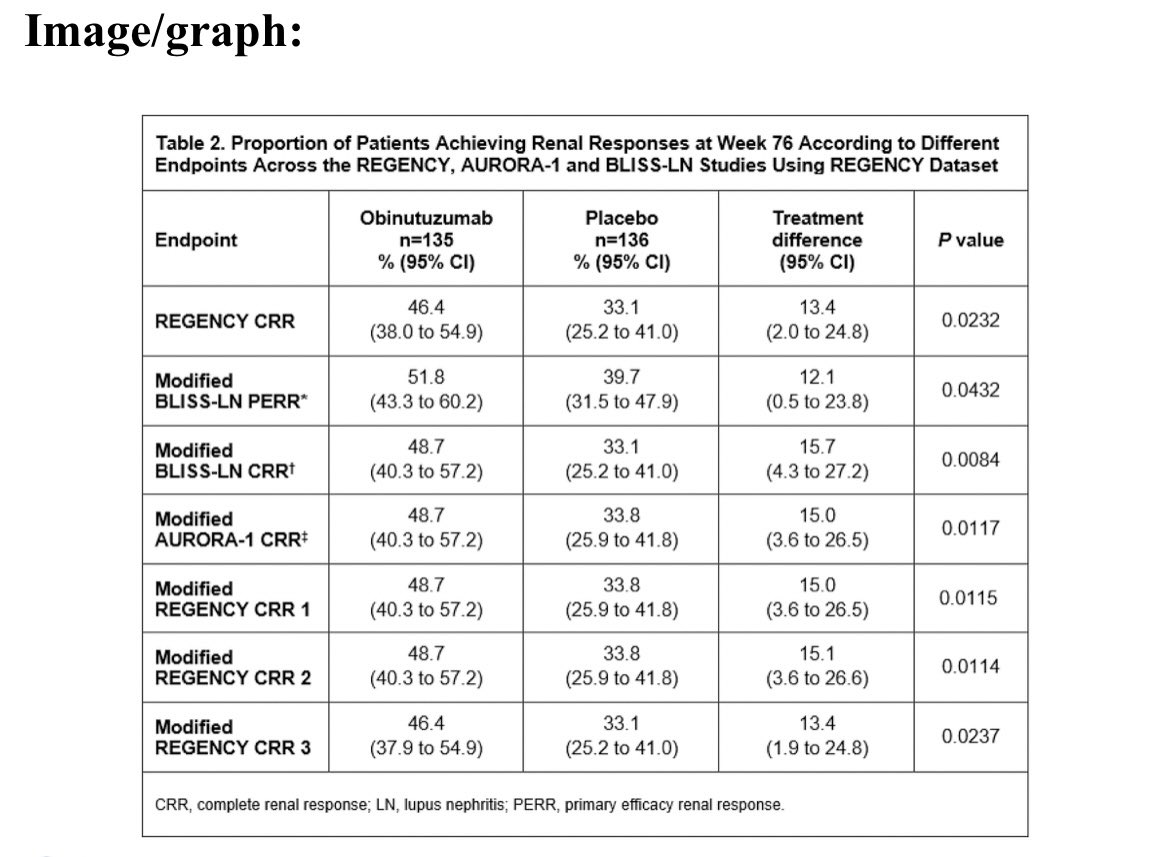
#EULAR2025 Abstr#OP0006. There is no standardised definition for complete renal response in #lupus nephritis. Post-hoc analysis of REGENCY showed Obinutuzumab + SOC was superior to PBO + SOC if endpoints for voclosporin and belimumab RCTs were used. Effect size ~13-16% @RheumNow https://t.co/q8MNAV8XF3

Janet Pope Janetbirdope
8 months 3 weeks ago
T2T in #gout w serum #uric #acid / #urate target
SAVES LIVES
Study of achievement of target 🎯
⬇️CVE and death
If not achieving Urate <360
T2T also reduced baseline #SUA
#EULAR2025 @rheumnow abst#OP0005 https://t.co/ePchJ4vqnU


Adela Castro AdelaCastro222
8 months 3 weeks ago
Secukinumab for PMR?
-post hoc analysis of the TitAIN study (phase 2 RCT on new onset/relapsing GCA) showed:
-Numerical reduction in patients experiencing PMR symptoms when treated with secukinumab compared to placebo.
-Safety profile was similar to the overall GCA study

Antoni Chan MD (Prof) synovialjoints
8 months 3 weeks ago
SPEED RCT: In early PsA with poor prognostic factors, PASDAS at 24wks:
•Early TNFi: 3.7
•Combo csDMARDs: 4.1
•Step-up csDMARDs: 4.7
Early TNFi beat step-up by -1.09 (p<0.001); combo csDMARDs also superior (-0.69, p=0.02). Early TNFi benefit sustained at 48wks. Abstract#OP0089 https://t.co/MpazF8BIr2


Antoni Chan MD (Prof) synovialjoints
8 months 3 weeks ago
In vitro, balinatunfib (TNFR1-selective inhibitor) preserved Treg expansion in CD4+ T cells co-cultured with IL-2 and memTNF (Treg 8.99 percent, p<0.0001), unlike adalimumab and etanercept which reduced Tregs by 27.5 to 41 percent. Confirms TNFR2 sparing with selective TNFR1 https://t.co/xaczz87Xrd


Md Yuzaiful Md Yusof Yuz6Yusof
8 months 3 weeks ago
#EULAR2025 Abstr#OP0002 Promising new mode of action therapy for #myositis. Phase 2 RCT of efgartigimod (FcRn-i; coformulated with recombinant PH20) showed improvement in TIS & other key endpoints vs PBO at Wk24. Injection reaction common (23%). Will proceed to Phase 3 @RheumNow https://t.co/SuMolfcDkS


Adela Castro AdelaCastro222
8 months 3 weeks ago
Phase 3 of POETYK-PsA-2:
-Deucravacitinib (oral TyK2i) met the primary endpoint of ACR20 at week 16 and was superior to placebo in multiple PsA domains.
-Clinical efficacy maintained until week 52.
-No major safety signals reported.
-Notably high placebo response rates.
Abstract
Wednesday was Day One at EULAR 2025 in Barcelona. Thousands from around the world gathered, eager to reunite at this international educational forum. Below are a few of my favorites from Day 1.

Jiha Lee JihaRheum
8 months 3 weeks ago
📊 In a 40-yr cohort (n=2818), RA pts had 2.3x ↑ risk of DVT (HR 2.26) & 1.6x ↑ risk of PE (HR 1.61) vs non-RA.
Risk ↑ with RF/CCP+, nodules (HR 2.9), BMI ≥30 (HR 1.9), & biologic use in year 1 (HR 2.4–2.9).
Remission = ↓ PE risk (HR 0.47)
📍OP0070
@RheumNow #EULAR2025

Jiha Lee JihaRheum
8 months 3 weeks ago
POS0627: RA patients aged ≥90 yrs can benefit from biologics/JAKi! In 3,600 pts, 84% of those ≥90 achieved low disease activity, 71% were on JAKi. But serious infections occurred in 21%—mostly pneumonia. Use w/caution in the oldest-old. #EULAR2025 @rheumnow









 Poster Hall
Poster Hall