Lupus

Janet Pope Janetbirdope
1 year 8 months ago
Can you ⬇️
#Glucocorticoids or #immunesuppressive in #SLE & change am’t of damage
From Asia Pacific #lupus collab
(Biologics not included)
Compared 7.5 mg #prednisone or less
Or DMARD
1/2 flared after LLDAS
⬇️dose didn’t prevent damage
#EULAR2024
@eular_org @RheumNow https://t.co/QmjEz87Cvw

Md Yuzaiful Md Yusof Yuz6Yusof
1 year 8 months ago
#EULAR2024 Cutaneous #lupus is variable & can be difficult to treat. Promising post-hoc data frm Phase 2 RCT showing Deucravacitinib (Tyk2-i) led to improvement & sustained CLASI-50 response in all subtypes (ACLE, SCLE, Chonic and Discoid) @RheumNow #EULARBEST https://t.co/ArAzdLYiYY


Md Yuzaiful Md Yusof Yuz6Yusof
1 year 8 months ago
#EULAR2024 OP0043 Data from UK BILAG-BR registry showed in n>100 patients w neuropsychiatric #lupus (NPSLE), over 2/3 improved with rituximab. NPSLE tended to occur with other #SLE features and with high level of organ damage @RheumNow https://t.co/q5olZgr6Pt


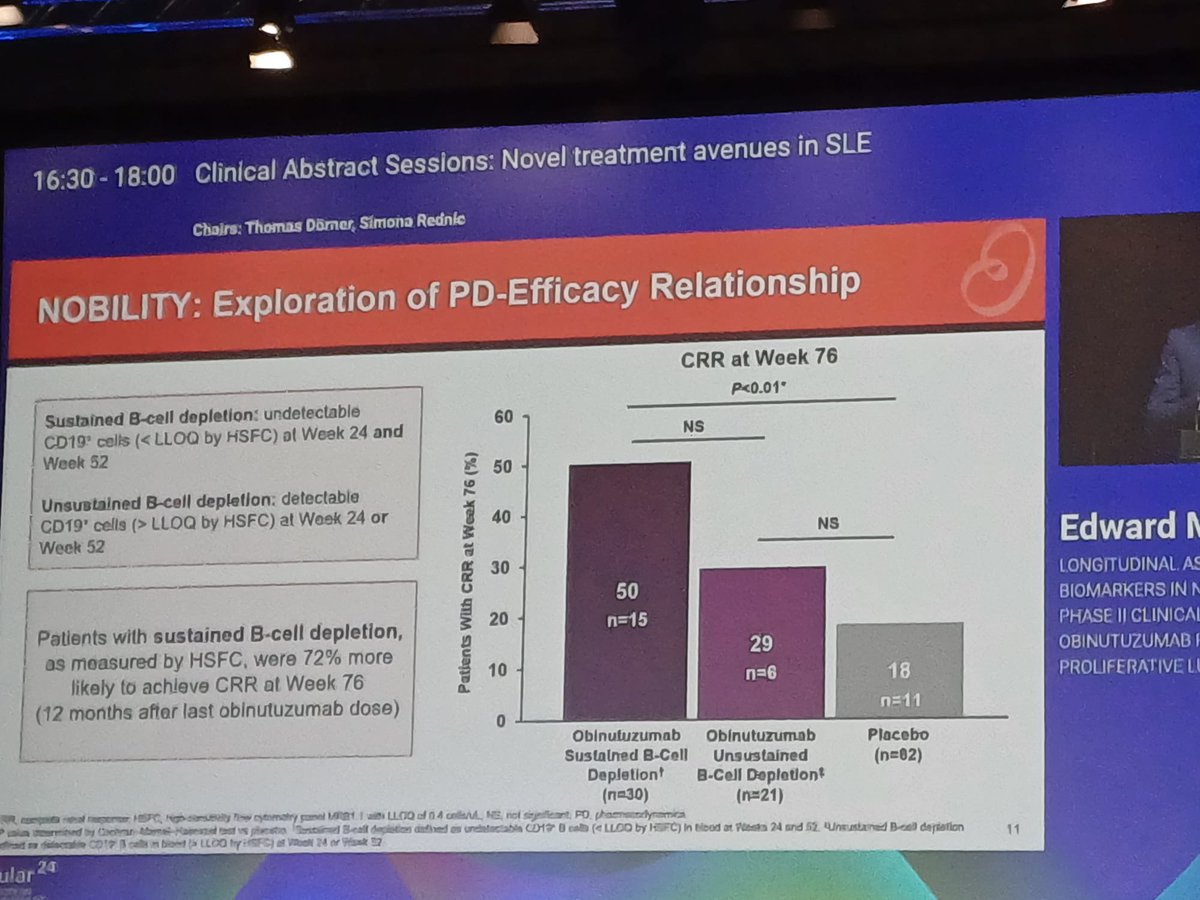
Md Yuzaiful Md Yusof Yuz6Yusof
1 year 8 months ago
#EULAR2024 OP0077 How can we improve Bcell killing and does deep depletion matter? Biomarkers from RCT showed Obinutuzumab led to profound circulatory depletion in #lupus nephritis & those w sustained depletion had better complete renal response than those who didn’t @RheumNow https://t.co/2KTZBR2njk

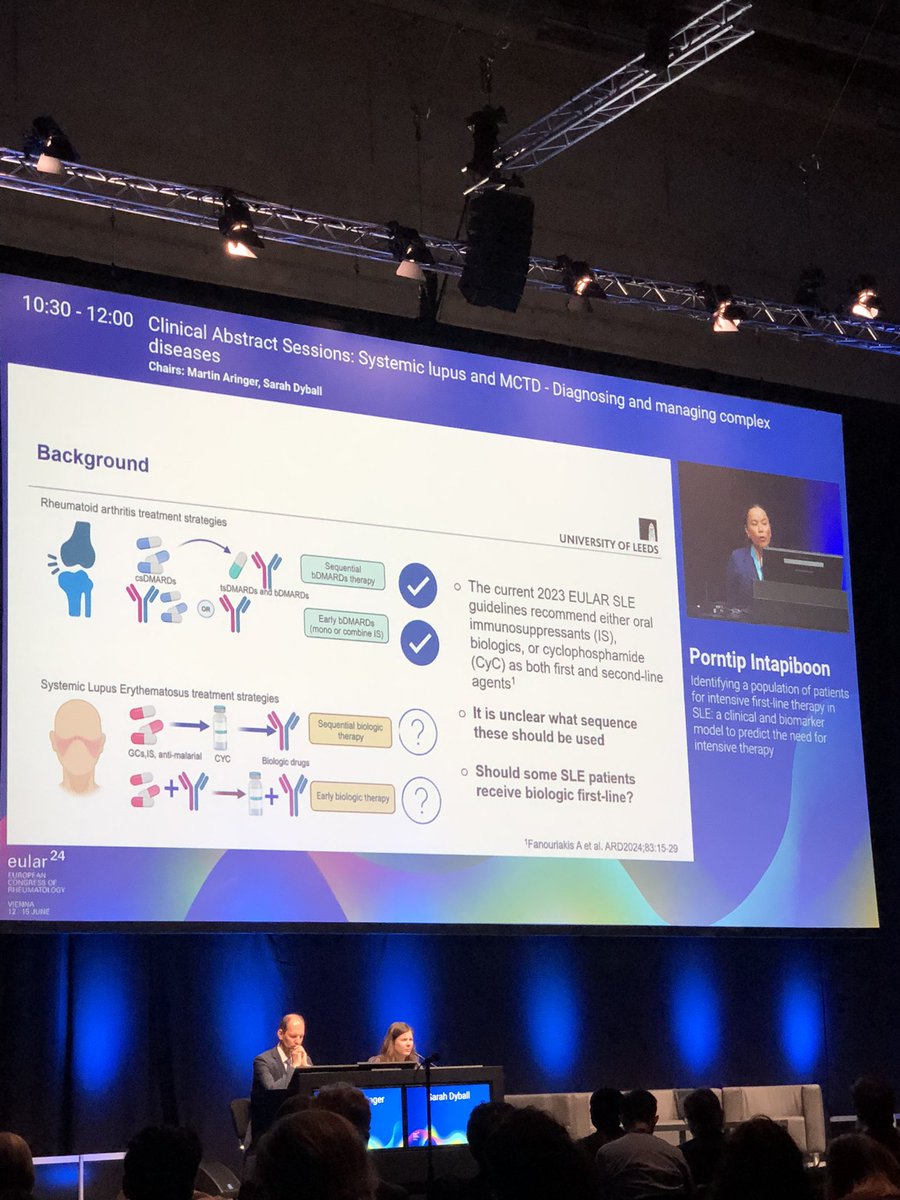
Janet Pope Janetbirdope
1 year 8 months ago
Who needs intensive therapy in #lupus
Intensive Rx in #SLE from 2 inception cohorts in Leeds
Either #Cyclo or #biologics
⬆️
If low complement
Within 2 yrs after #dx
#SLEDAI >9
AntiRo+
OP0187
#EULAR2024 @RheumNow @eular_org https://t.co/oKhwMmHhSs

Janet Pope Janetbirdope
1 year 8 months ago
3 facts in #lupus to make you think 🤔
💧biomarkers in #SLE blood & #urine May predict #activity
OP0255
#Membranous #nephritis is serious in SLE @ZahiTouma POS0732
#prednisone dose irrelevant for #damage if in LLDAS -no diff between 7.5mg vs 5mg OP0124
#EULAR2024 @RheumNow

Janet Pope Janetbirdope
1 year 8 months ago
#Hope for patients with #active #SLE
The next hurdle is getting through
▶️Phase3 #RCTs
And
▶️Obtaining drug reimbursement so patients can get access to effective
#lupus #Rx
#EULAR2024
@RheumNow @eular_org https://t.co/sGzQXO4A2r
Wednesday was Day One at EULAR 2024 in Vienna. While the day was a slow start, the poster halls and auditoriums quickly filled with thousands of rheumatologists, eager to reunite at this international educational forum. Below are a few of my favorites from Day 1.

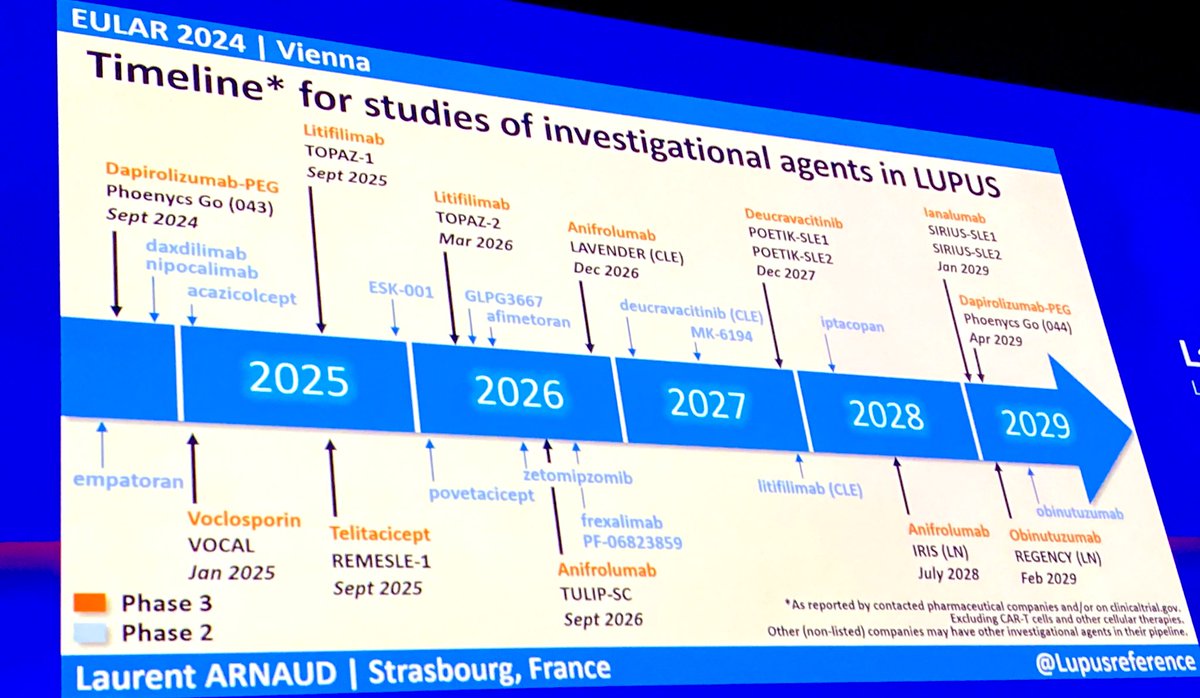
Dr Gurdeep S Dulay gurdeep_dulay
1 year 8 months ago
#EULAR204
Encouraging Timeline for future potential treatments for #SLE #Lupus
@LUPUSUK @LupusMadrid @Lupus_Chat @LupusEurope https://t.co/SLKc0XCtFq
Large language models, such as ChatGPT, are advanced systems trained on vast amounts of text data, far exceeding what a human can read in a lifetime, to understand and generate human-like language. With these tools at our disposal, they are inevitably making their way into healthcare. One notable example is response optimization for SLE patients, as highlighted in abstract #0989.

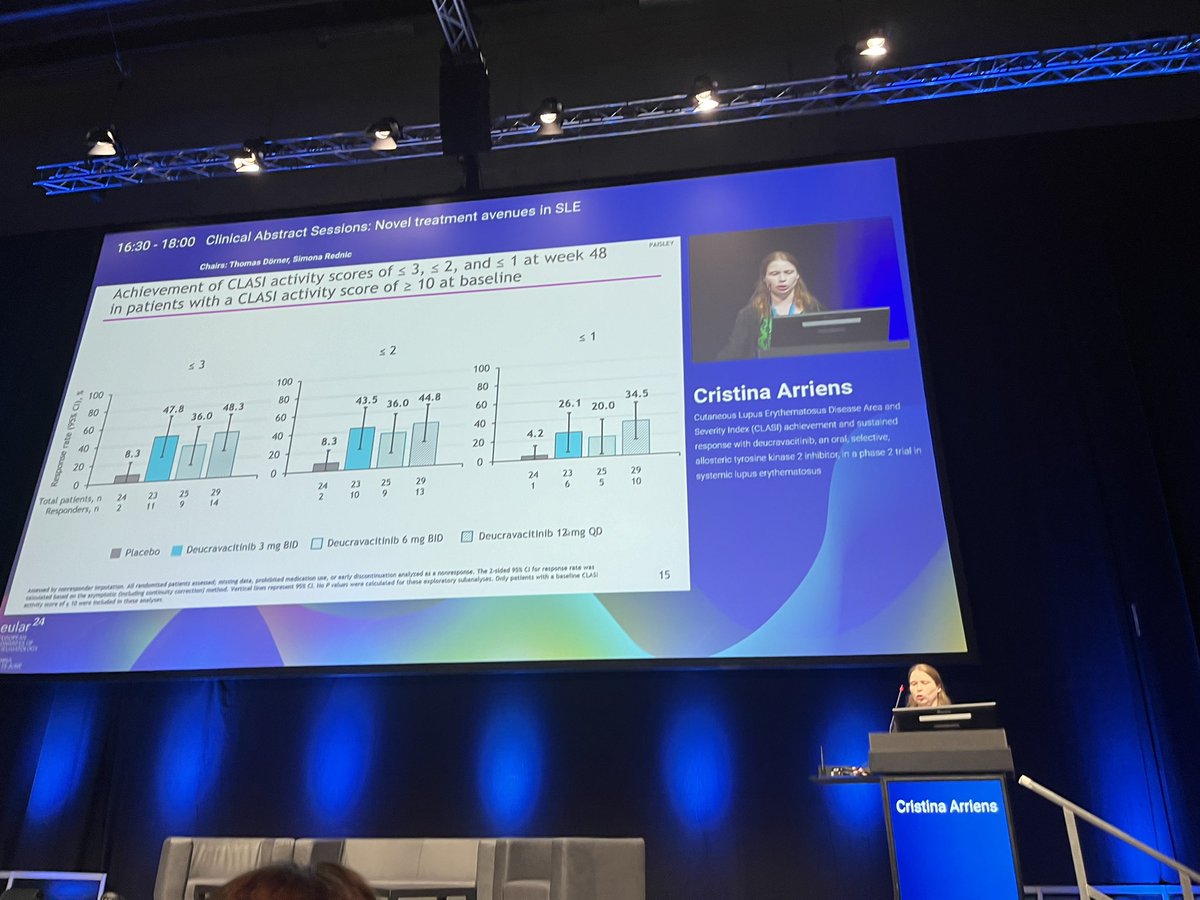
TheDaoIndex KDAO2011
1 year 8 months ago
OP0048 Impressive CLASI (lupus skin index score) with #deucravacitinib - data presented by Dr C Arriens #EULAR24 @RheumNow #WiR https://t.co/EuQdwuolN6

TheDaoIndex KDAO2011
1 year 8 months ago
Novel B cell depleters! Bispecific T cell engagers (BiTEs) being studied for #SLE & #RA as a way to get CAR-T cell like responses but more accessible w/less risk for CK release syndrome. #EULAR24
T cell-engaging therapies — BiTEs and beyond | Nature Reviews Clinical Oncology

Md Yuzaiful Md Yusof Yuz6Yusof
1 year 8 months ago
#EULAR2024 Please find my article on a preview of interesting abstract to look out for for #lupus #SLE therapies at this year’s congress @RheumNow
https://t.co/TEtrDWq3BF https://t.co/1zIIxsaeL2















 Poster Hall
Poster Hall