JAK/TYK2
Psoriatic arthritis is currently experiencing a burgeoning selection of treatment options. While this is a very welcome development in a disease which has had less treatment options compared to RA, it leaves us with a difficult conundrum: which agent to choose for an individual patient.

David Liew drdavidliew
4 years 2 months ago
Will a FDA black box warning change your JAKi practice?
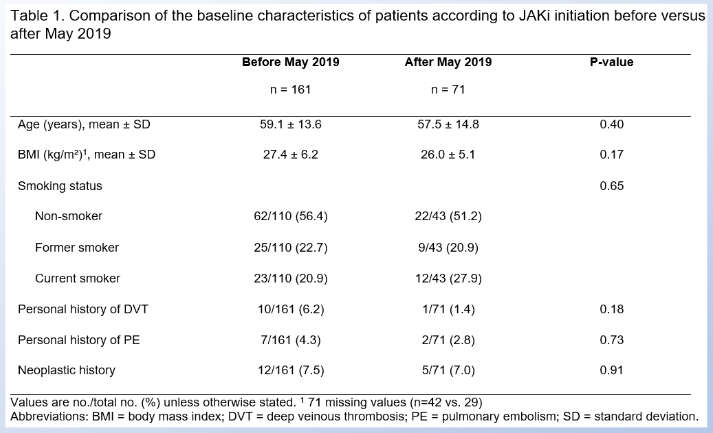
In May 2019, the EMA put out a warning about VTE with tofacitinib.
In this French cohort, did it change the kind of pts they initiated on JAKi?
no, not it did not😊
A window into the US future?
#ACR21 ABST1245 @RheumNow https://t.co/QI95E1gepg

Mrinalini Dey DrMiniDey
4 years 2 months ago
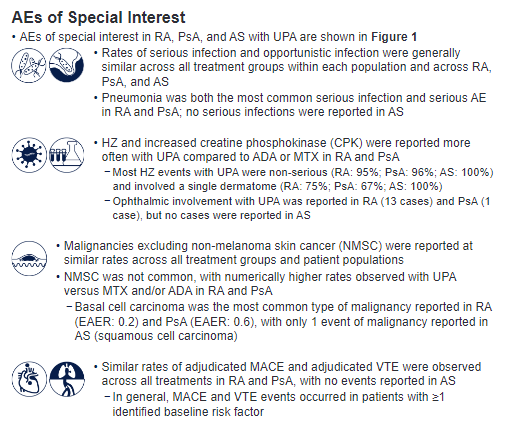
Safety profile of #upadacitinib in #RA, #PsA, #AnkylosingSpondylitis
👉🏼AE rates similar across UPA, ADA & MTX in RA, and UPA & ADA in PsA
👉🏼No new safety risks with long-term use
👉🏼#Herpeszoster & incr CPK more common w/ UPA vs ADA/MTX
Abs#1691 #ACR21 @RheumNow https://t.co/tKYi65kB5r
The third day of ACR 2021 took a big leap in online content. Here is a compilation (with links) of presentations were the “ACRBest” as seen by our RheumNow faculty.

Pedro Castillo _Castillo_Pedro
4 years 2 months ago
Upadacitinib 56-wk efficacy/safety (mod to severe PsA)
🔹Comparable or ⬆️efficacy vs adalimumab
🔹Efficacy maintained, and 15mg vs 30mg doses similar at 56wks
🔹No new safety findings @ 56wks
🔹🚫inc risk VTE, MACE, cancer vs ada
https://t.co/NlKvBrwDxB
#ACR21 Abst#1345 @RheumNow

David Liew drdavidliew
4 years 2 months ago
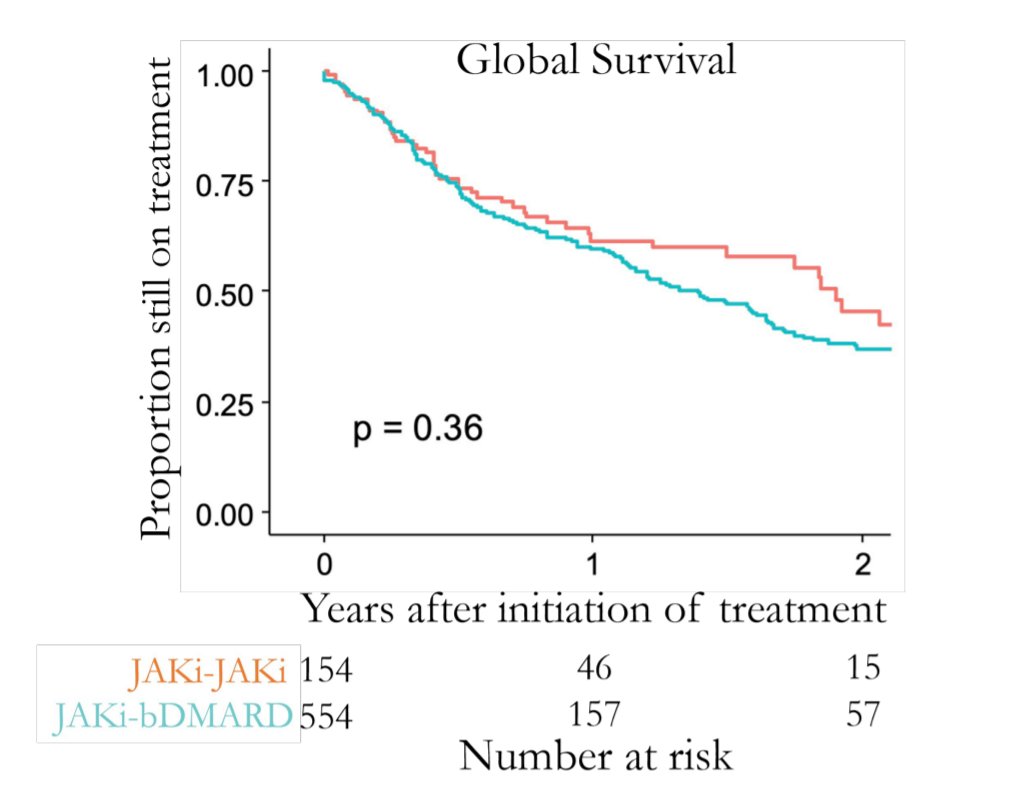
Interesting question came up: why would you change a RA patient from JAKi to JAKi?
The main reason I can think of would be if the first JAKi worked a bit (but not enough to continue, or AEs got in the way).
That would clearly favour to better survival
#ACR21 ABST1442 @RheumNow https://t.co/kDiJVbJfit

Richard Conway RichardPAConway
4 years 2 months ago
JAK-pot registry-based study. Following JAKi failure should you switch mode of action or go to second JAKi? Both seem equally effective in terms of drug retention. Didn't matter if switch for efficacy or adverse event. Abstr#1442 #ACR21 @RheumNow https://t.co/QEBB3flArC

Eric Dein ericdeinmd
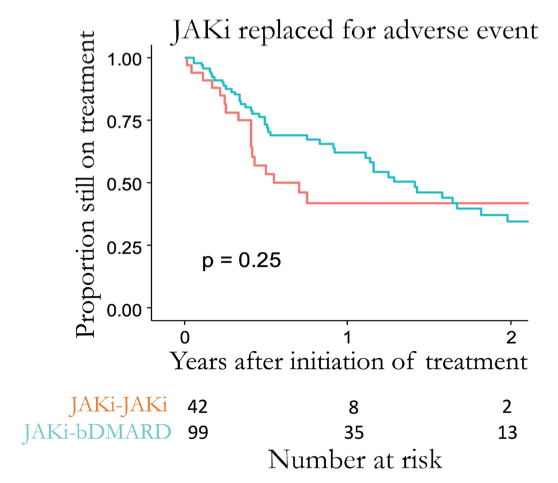
4 years 2 months ago
#ACR21 Abs#1442: JAKpot - Cycle JAK or switch to bDMARD after JAK failure?
▶️No difference between cycle vs bDMARD group
▶️Non-signif trends: cycled JAK better for inefficacy to 1st JAK, bDMARD better for adverse effects
@Rheumnow https://t.co/6UNUVh5ZW8 https://t.co/upfI6tDH1p

Aurelie Najm AurelieRheumo
4 years 2 months ago
First JAKi failure: What's next? Wanna find out? It's here
https://t.co/o5CE1MBzUu
Abstr#1442 #ACR21 @RheumNow https://t.co/f0sdPn5mcZ

Aurelie Najm AurelieRheumo
4 years 2 months ago
⭐️ Wanna catch up with the latest news on JAKi safety trials presented at #ACR21? Our @Rheumnow interview with @ErnestChoy1 is out. Link below
https://t.co/zYb4rXcw1x https://t.co/i39xXnfC3Q

Robert B Chao, MD doctorRBC
4 years 2 months ago
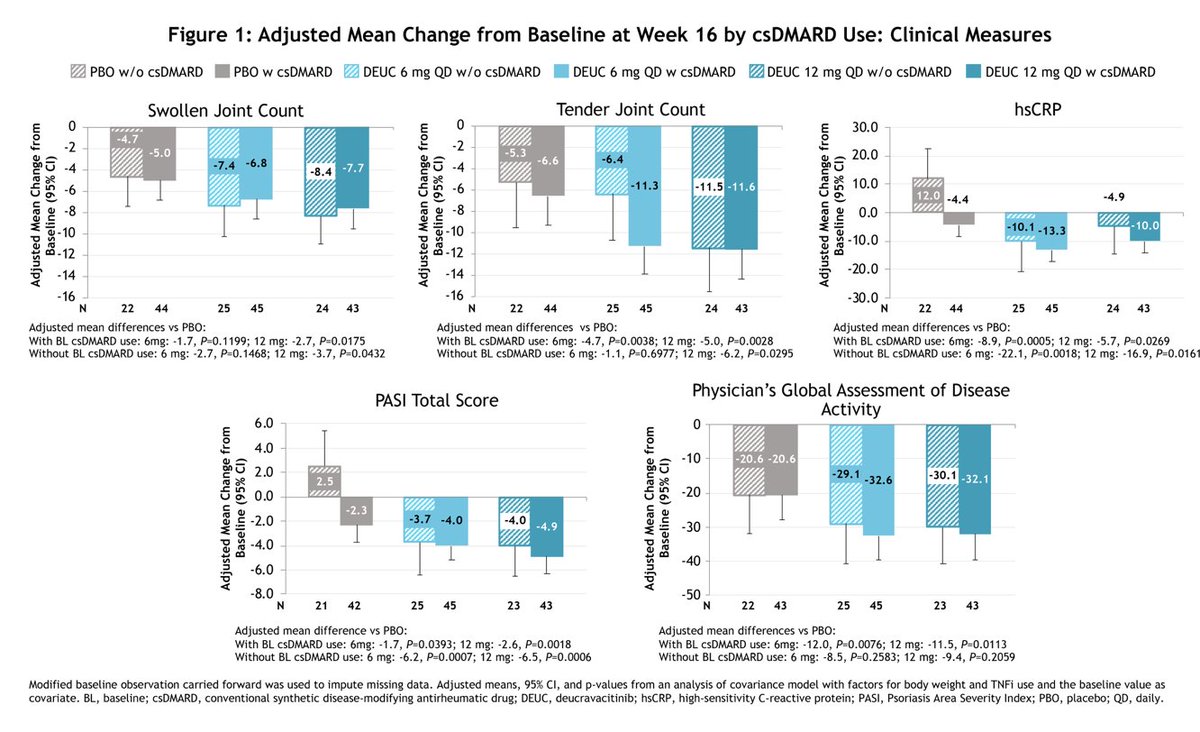
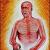
Post-hoc analysis of Deucravacitinib (TYK2i) for tx of PsA showed similar efficacy in pts with and without background csDMARD use.
⭐️>60% w/ background csDMARD use, majority of which was MTX
⭐️no difference in AE
#ACR21
Abs#1352
@RheumNow
https://t.co/nIJT7rNkJP https://t.co/bq0x1Ywguo














 Poster Hall
Poster Hall