Drug Safety

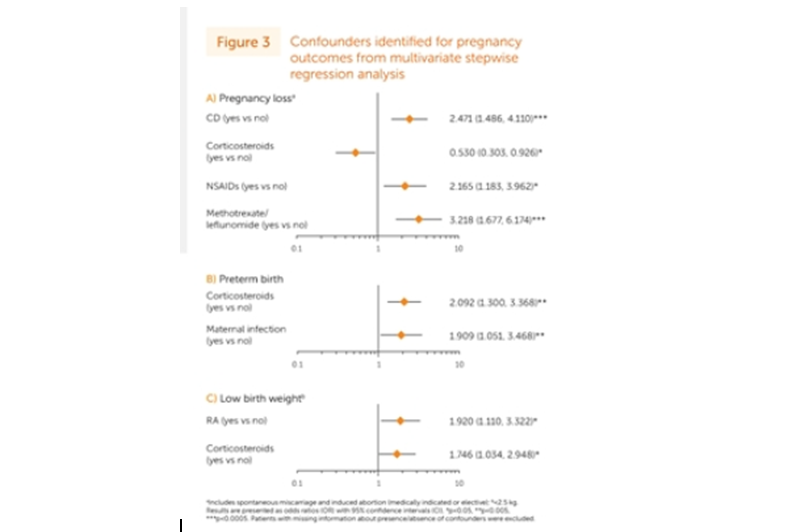
#POS0022 prosp study of CTZ pregnancies: 1392 preg w/1425 outcomes @UCBUSA database
88.4% live births, 10.5% preg loss, 0.8% still birth
2.4% congen malform (w/2.1% major)
Preg loss⬅️Crohn's, NSAIDS, MTX
Preterm labor⬅️GCs, maternal infx
⬇️birth wt⬅️RA & GCs @rheumnow #EULAR2021 https://t.co/qnNcDIz8Ge

2 years 11 months ago
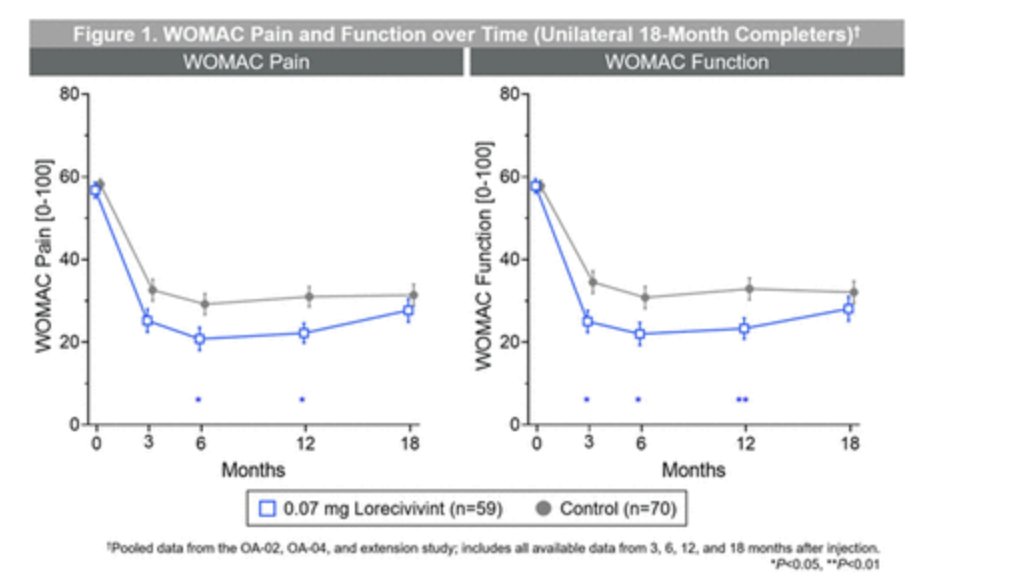
A single dose of LORECIVIVINT IA (Wnt pathway modulator) in Phase 3 study in knee OA: mild improvement of WOMAC pain -8.16[-15.60, -0.71] P=0.032 and function -9.47[-17.09, -1.84] P=0.015 at 6 and 12months with no particular safety signal. #POS0278 #EULAR2021 @RheumNow https://t.co/5qQE6MPjnU

2 years 11 months ago
Poster #POS1031 pooled VOYAGE 1&2 and DISCOVER 1&2, GI-related SAE rates low. No uveitis, opportunistic infections, or new onset/exacerbation of IBD in GUS-treated pts. No new safety concerns were identified through 1-year follow up. #EULAR2021 @RheumNow https://t.co/7NxeOu6FLW

2 years 11 months ago
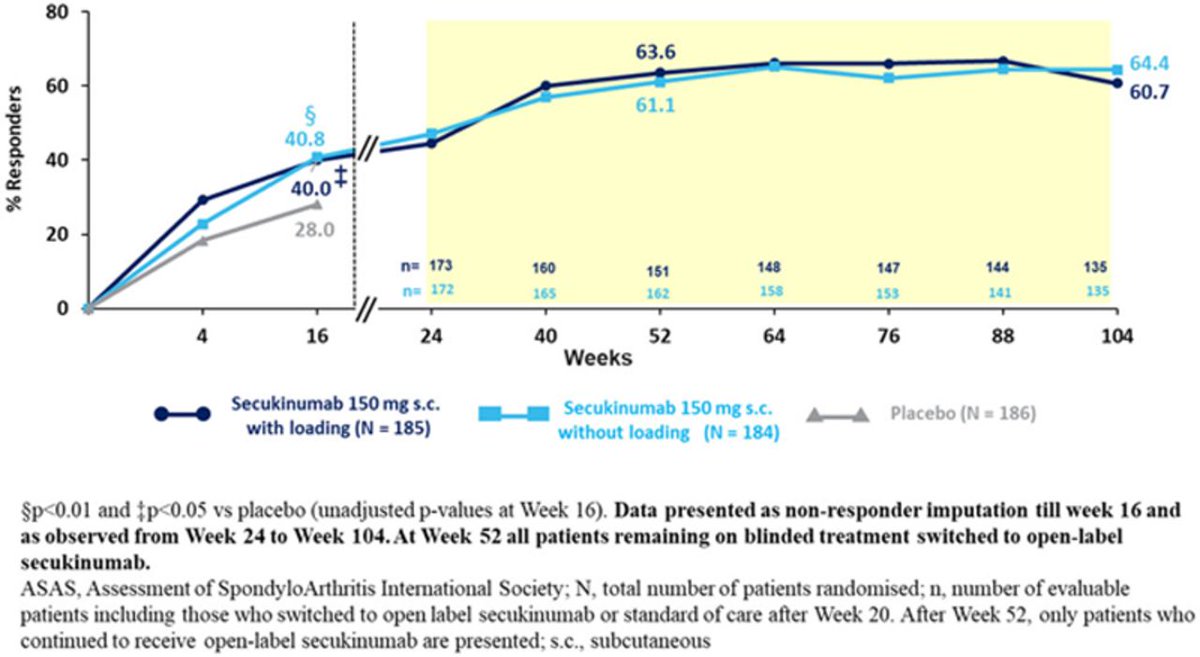
PREVENT: SEC 150 mg showed durable improvement in nr-axSpA through 2 yrs, 438 pts. No new or unexpected safety signals. Poster #POS0900 #EULAR2021 @RheumNow https://t.co/NU1EO9zktn https://t.co/LP3hkZodhi

2 years 11 months ago
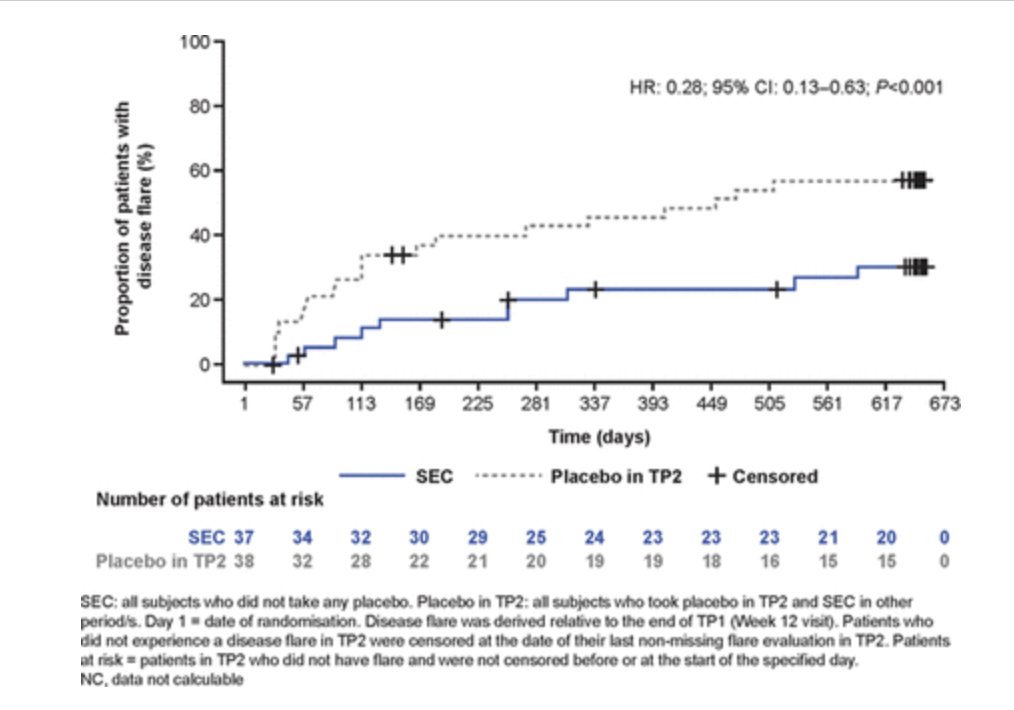
JUNIPERA trial: open label Secukinumab in ERA and JPsA: 90.4% JIA ACR 30 and 69.9% JIA ACR 70 W12 and > time to flare W104, 72% risk of flare reduction SEC vs PBO (HR: 0.28 95CI: 0.13–0.63 P<0.001). No particular safety signal.
#LB0002 @Rheumnow #EULAR2021 https://t.co/PJ9NYi25UI

2 years 11 months ago
#EULAR2021 and EULAR IQ
Dr Jack Cush reviews highlight presentations from the first 3 days of EULAR 2021.
https://t.co/BkHjhOUqNU https://t.co/4JS1yGiJHg


2 years 11 months ago
KEEPsAKE 2: Risankizumab RCT in Bio-IR or csDMARD-IR active PsA. Primary endpoint ACR20 W24 met with 51.3% vs 26.5% in PBO group (P < .001) and ACR50 26.3% vs 9.3%. No particular safety signal.
#OP0228 @Rheumnow #EULAR2021 https://t.co/HZv8qoc4sz
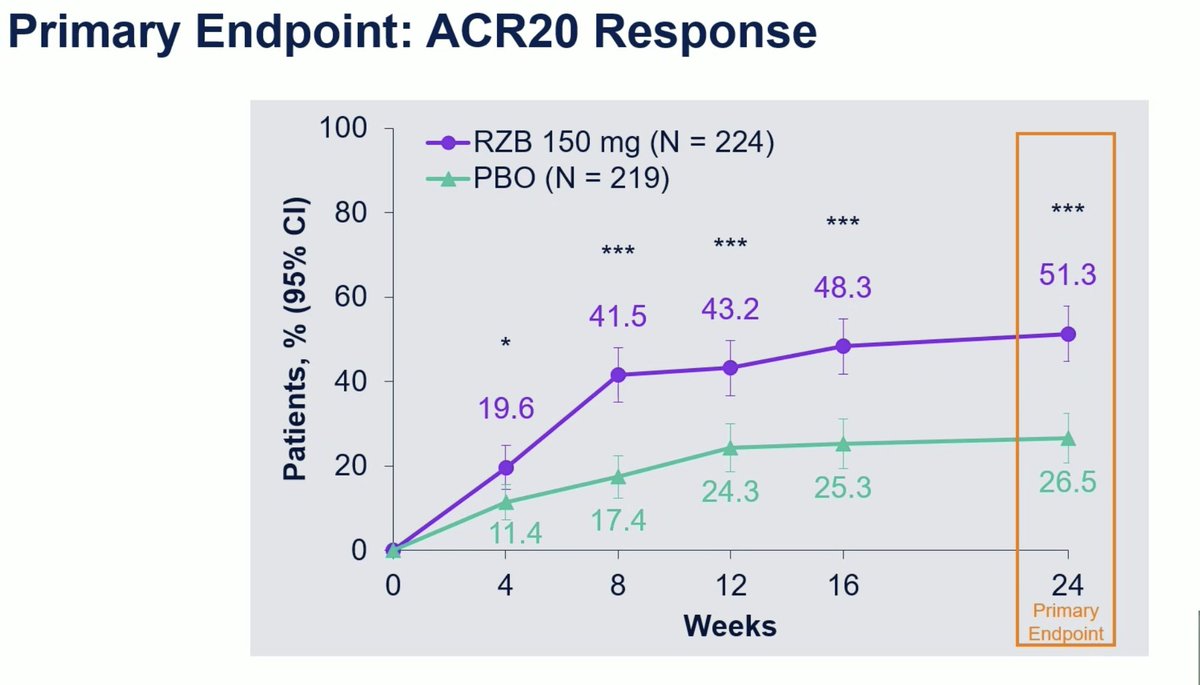

2 years 11 months ago
COSMOS study: Guselkumab RCT in PsA pts TNFi-resistants (1 or 2) 44.4% of GUS vs 19.8% of PBO pts achieved ACR20 at w24 (p<0.001) and GUS > PBO for most secondary endpoints. Tolerance: 18% vs. 20% infections/SI.
#OP0230 @Rheumnow #EULAR2021 @DrLauraCoates https://t.co/fFle2OR8KM
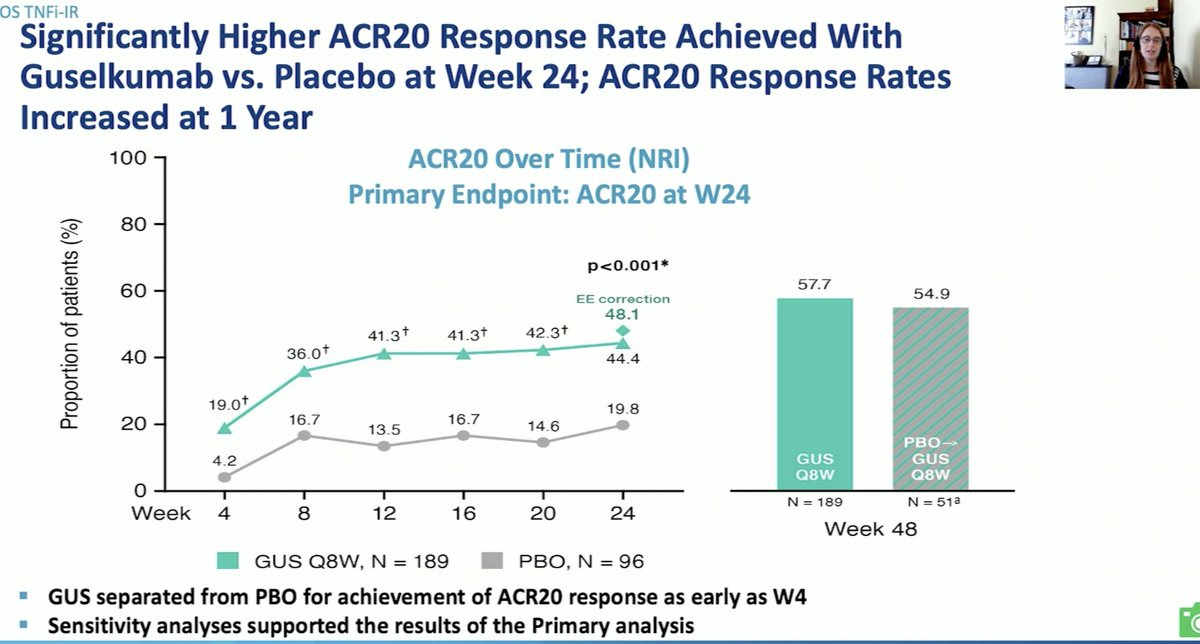

2 years 11 months ago
Serious infections and BioDMARDs: what is the confounding factor?
BSRBR-RA: 33,916 RA treated w/ BioDMARDs with 62,532 years of follow-up, 2036 SI. HR of SI no longer different among BioDMARDs when adjusted for the line of therapy! #OP0241@Rheumnow #EULAR2021 https://t.co/UG9WrXP7RD
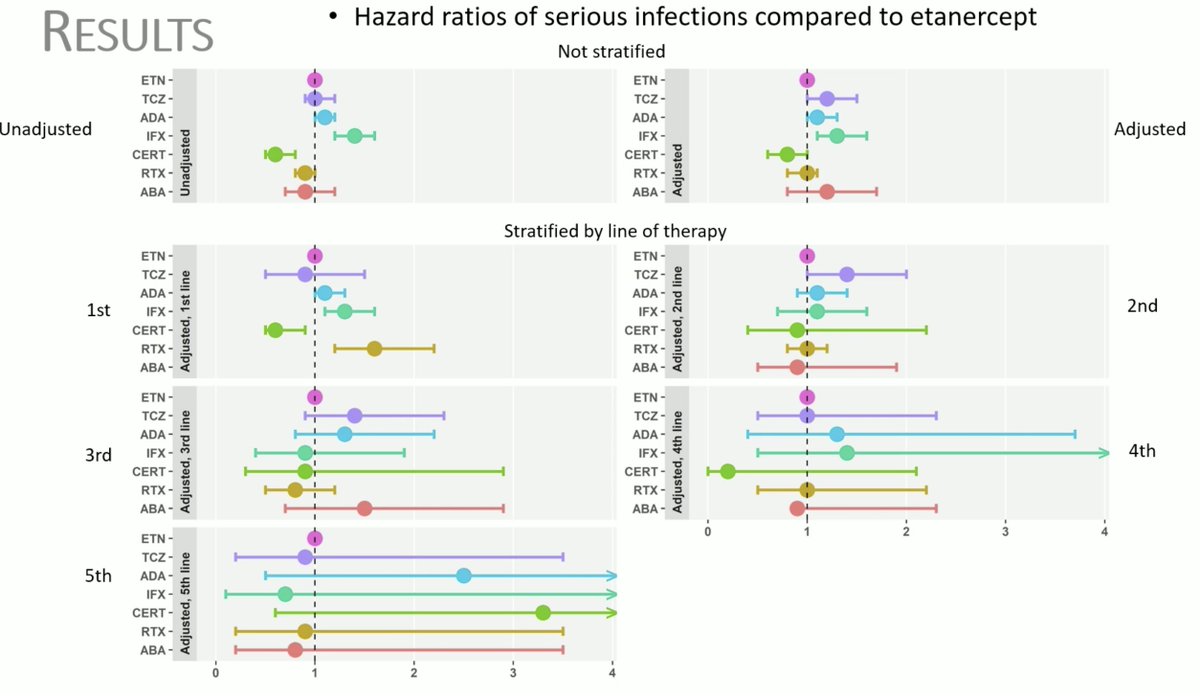
Check out this compilation of our EULAR 2021 Day 2 broadcasts below.
You can also follow the EULAR 2021 RheumNow podcasts on iTunes and Soundcloud.com
Listen by Clicking below:
1.…











 Poster Hall
Poster Hall