Psoriatic arthritis

2 years 11 months ago
RheumNow’s expanded coverage of the #EULAR2021 Annual meeting is sponsored in part by Novartis, Bristol Myers Squibb, and Janssen. All content chosen by RheumNow & its Faculty.

2 years 11 months ago
Which patients with psoriasis are at risk to develop PsA?
The potential transition from psoriasis (PsO) to psoriatic arthritis (PsA) has not been a strong focus for several years but is gaining momentum.
https://t.co/5sj5HSnke3 https://t.co/NwoIoDH5rC

2 years 11 months ago
JUNIPERA - secukinumab in enthesitis related arthritis and juvenile PsA
⭐️achieved JIA ACR response compared to placebo
⭐️⬇️time to flare
⭐️75% with resolution of enthesitis
⭐️0 deaths, AE - minor infections, GI issues, headache
Abs#LB0004
#EULAR2021 @RheumNow

2 years 11 months ago
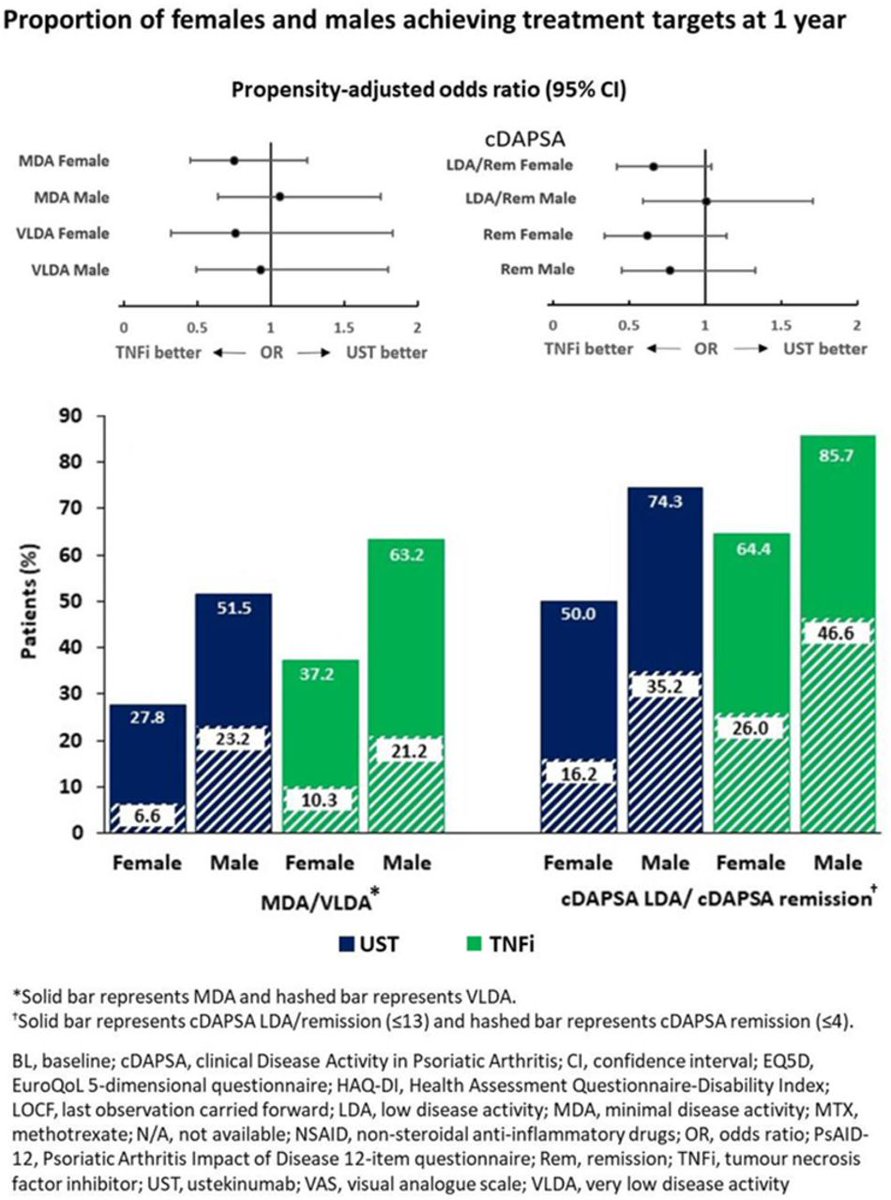
Real-world data from PsABio: 🚺generally start biologics in a worse PsA state than🚹. Although treatment improvements were similar between sexes, 🚺remained in worse health at 1 yr, and stopped/switched bDMARD earlier. Abstract #OP0232 #EULAR2021 @RheumNow https://t.co/oIGu0HzisQ https://t.co/uLnYOq8ypx

2 years 11 months ago
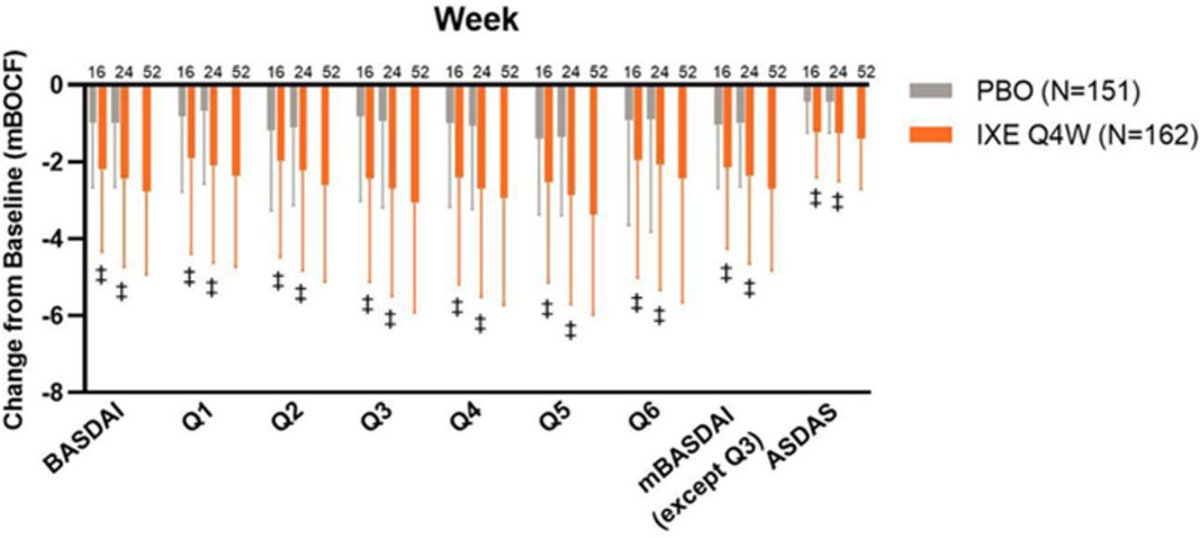
SPIRIT-P1&P2: IXE is effective in⬇️axial symptoms & improving QoL in active PsA pts with axial involvement. Abstract #POS1045 #EULAR2021 @RheumNow https://t.co/GC78Lmp9cH https://t.co/WfaUqIRrY6

2 years 11 months ago
#POS1069 Quite promising results of the use of an easy to use quick quantitative CRP assay to be included in the DAPSA to facilitate immediate decision making. @ProftDr
In 98% identical disease activity classification than with #DAPSA
#EULAR2021 @RheumNow https://t.co/Rt8fKvKBC4


2 years 11 months ago
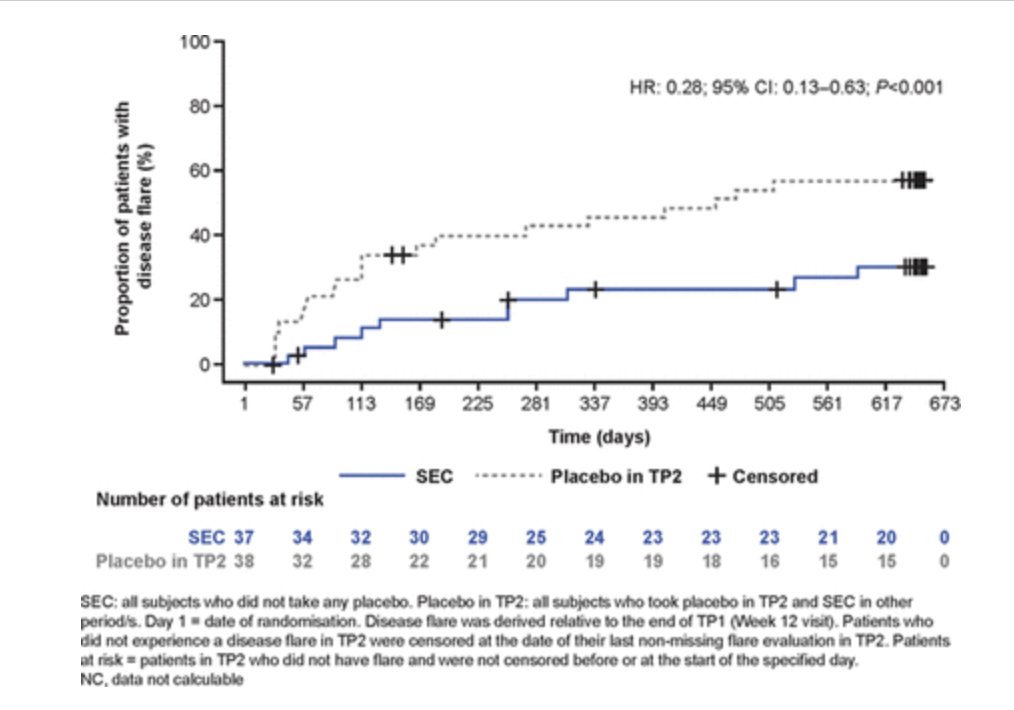
JUNIPERA trial: open label Secukinumab in ERA and JPsA: 90.4% JIA ACR 30 and 69.9% JIA ACR 70 W12 and > time to flare W104, 72% risk of flare reduction SEC vs PBO (HR: 0.28 95CI: 0.13–0.63 P<0.001). No particular safety signal.
#LB0002 @Rheumnow #EULAR2021 https://t.co/PJ9NYi25UI

2 years 11 months ago
#EULAR2021 and EULAR IQ
Dr Jack Cush reviews highlight presentations from the first 3 days of EULAR 2021.
https://t.co/BkHjhOUqNU https://t.co/4JS1yGiJHg


2 years 11 months ago
RheumNow’s expanded coverage of the #EULAR2021 Annual meeting is sponsored in part by Novartis, Bristol Myers Squibb, & Janssen. All content chosen by RheumNow & its Faculty.

2 years 11 months ago
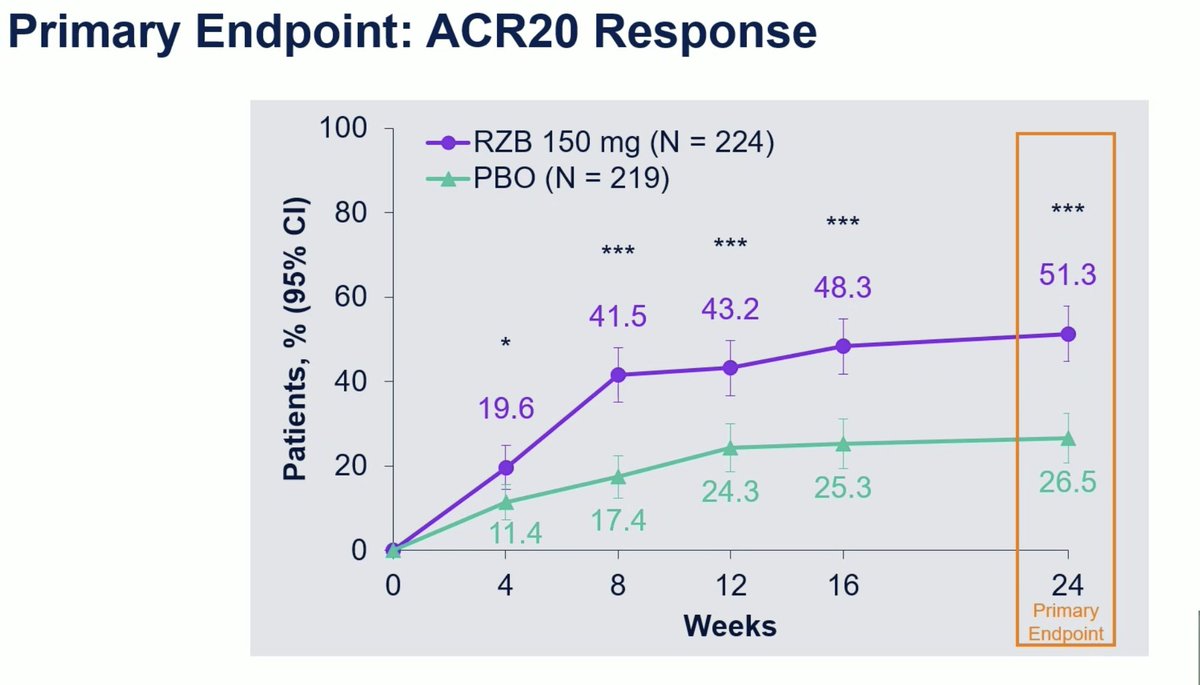
KEEPsAKE 2: Risankizumab RCT in Bio-IR or csDMARD-IR active PsA. Primary endpoint ACR20 W24 met with 51.3% vs 26.5% in PBO group (P < .001) and ACR50 26.3% vs 9.3%. No particular safety signal.
#OP0228 @Rheumnow #EULAR2021 https://t.co/HZv8qoc4sz









 Poster Hall
Poster Hall