Anti-Rheumatic Rx

David Liew drdavidliew
3 years 7 months ago
Once we add adalimumab in MTX-IR RA, can we dial down MTX & still keep control?
New data - now in East Asian pts (with already low MTX) says often yes, without ⬆️ADAb.
Obviously RA control 1st/2nd/3rd, but prob doesn’t have to be MTX 20 forever in everyone #EULAR2022 @RheumNow https://t.co/aZ0bsgY7dC

TheDaoIndex KDAO2011
3 years 7 months ago
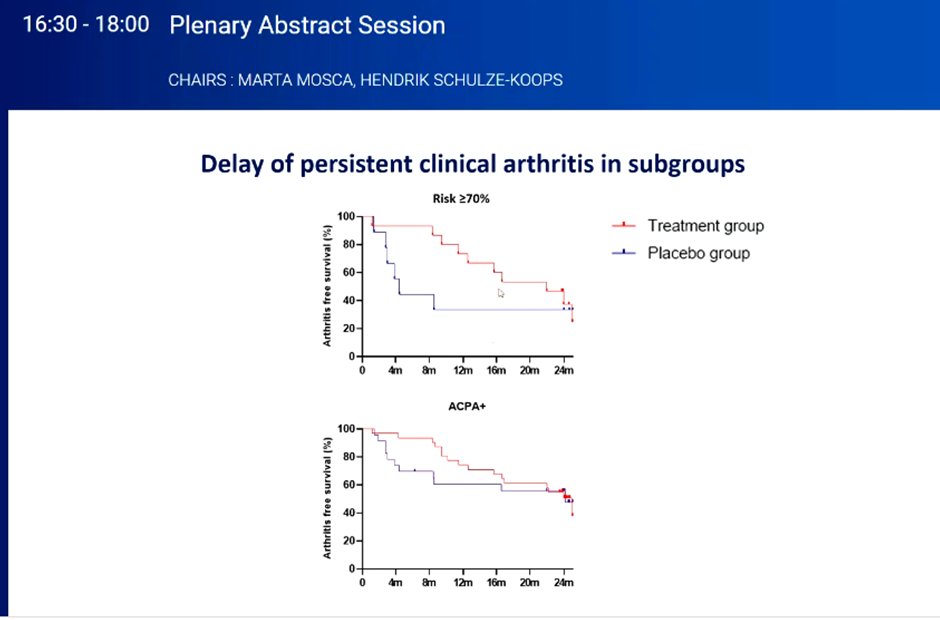
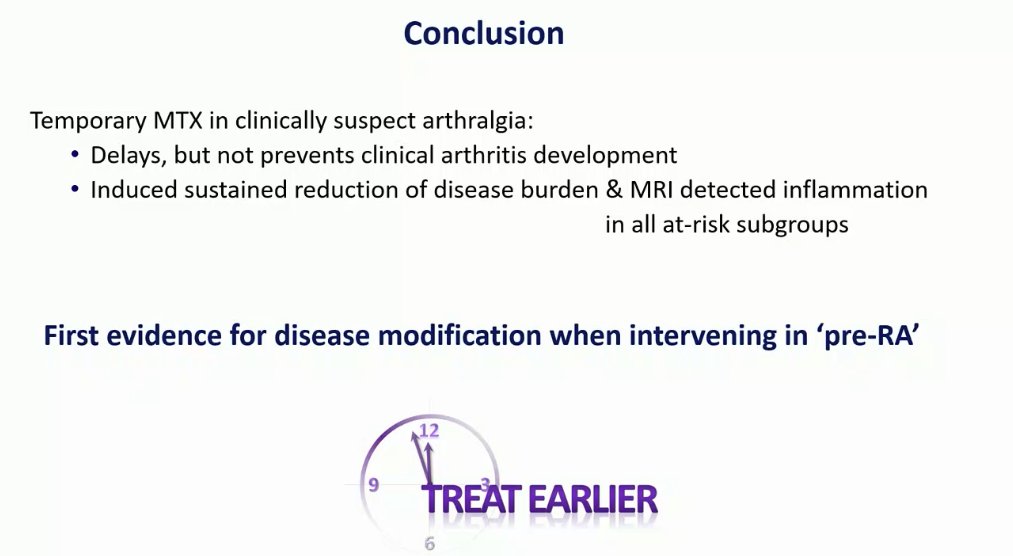
Pre-clinical RA TREAT EARLIER Trial: #Plenary OP0070
RDBPCT eval temporary MTX vs PCB in 136 pts with arthralgias >2 weeks at risk for RA (MRI hand/forefoot w/subclinical inflammation) followed for 2 yrs; 1/3 +ACPA. Rx 1 yr can delay but won’t prevent RA. #EULAR2022 @rheumnow https://t.co/v1GWOBFstz

Eric Dein ericdeinmd
3 years 7 months ago
#EULAR2022 TREAT-EARLIER OP0700
Arthralgia + subclinical inflammation in MRI without RA, treated with MTX + depo steroid injection
▶️In High risk patients, delayed development of synovitis, but does not prevent in long-term
▶️Reduction in disease burden on MTX Rx
@RheumNow https://t.co/SKlqMQQTui

Richard Conway RichardPAConway
3 years 7 months ago
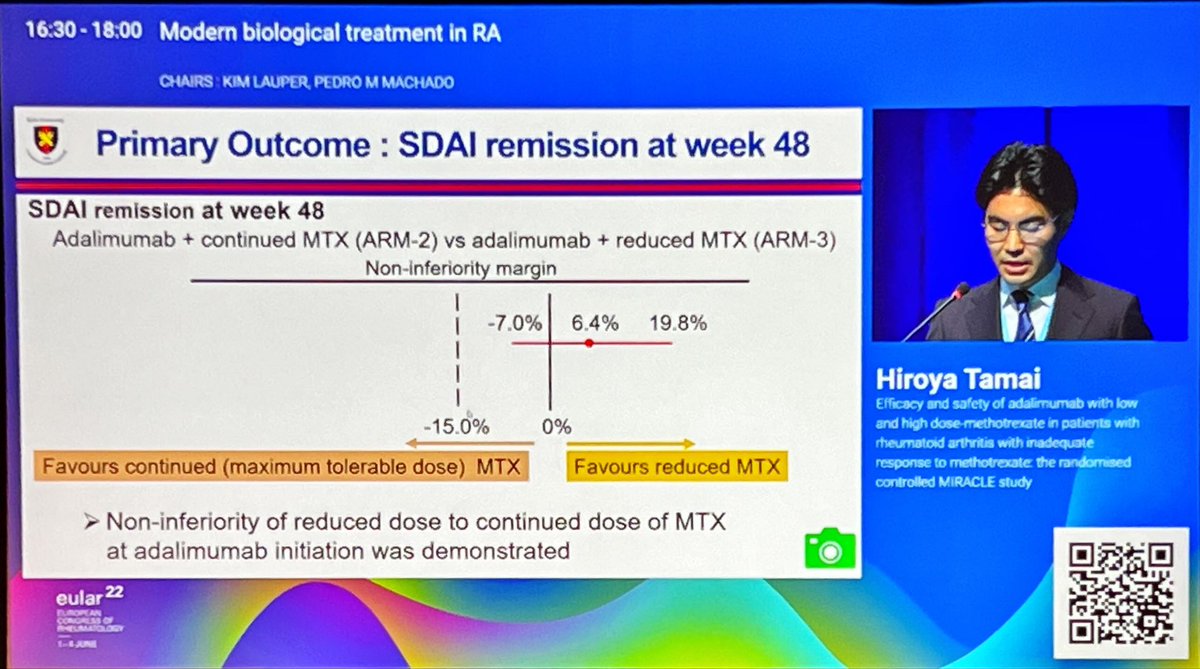
MIRACLE (love it!) study. High (max tolerated up to 25mg) vs low dose (6-8mg) MTX in combo with ADA in MTX-IR. Low dose non-inferior with better safety. Japanese population so may not be generalisable. @RheumNow #EULAR2022 OP0062 https://t.co/l3CfzzM7mi https://t.co/lkjuoqLY56
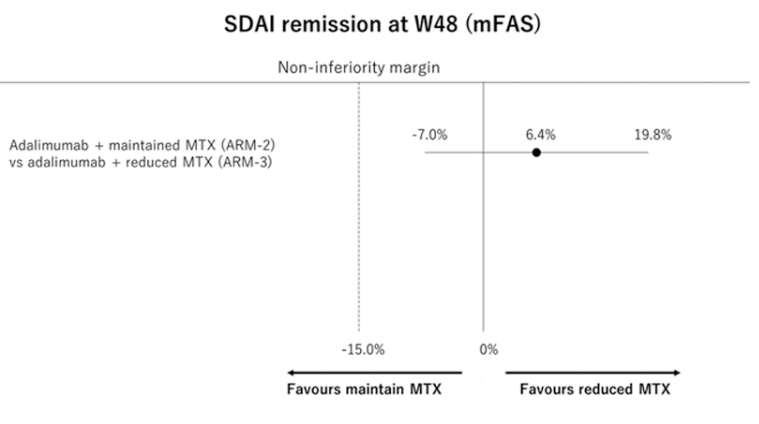

Richard Conway RichardPAConway
3 years 7 months ago
Krijbolder et al on MTX to prevent RA in clinically suspect arthralgia. No effect on arthritis free survival (80 vs 82%). Some delay in RA onset in high-risk group. But there was a sustained improvement in symptoms in MTX group @RheumNow #EULAR2022 OP0070 https://t.co/0CPIP51yQd https://t.co/kWNmw1YzrH
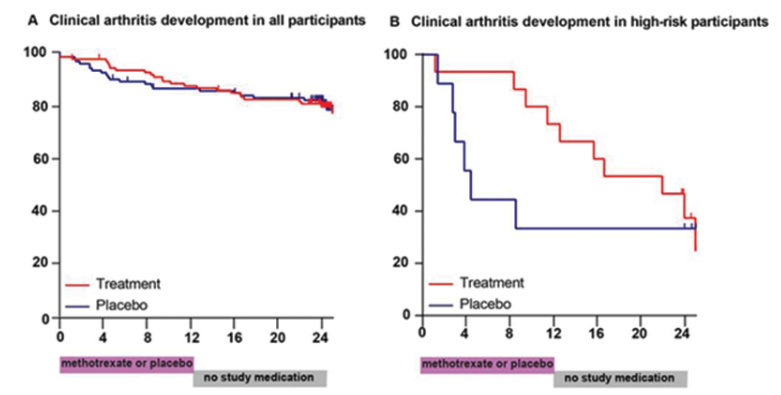

Richard Conway RichardPAConway
3 years 7 months ago
NORD-STAR substudy looking at anticoagulant effects. Important as RA assoc. ⬆️DVT/PE. DMARDs improve haemostatic imbalance, with bDMARDs stronger effect than csDMARD (may relate to better disease control with this) @RheumNow #EULAR2022 OP0059 https://t.co/TKwAZMOZjY https://t.co/IazrfD7z7k
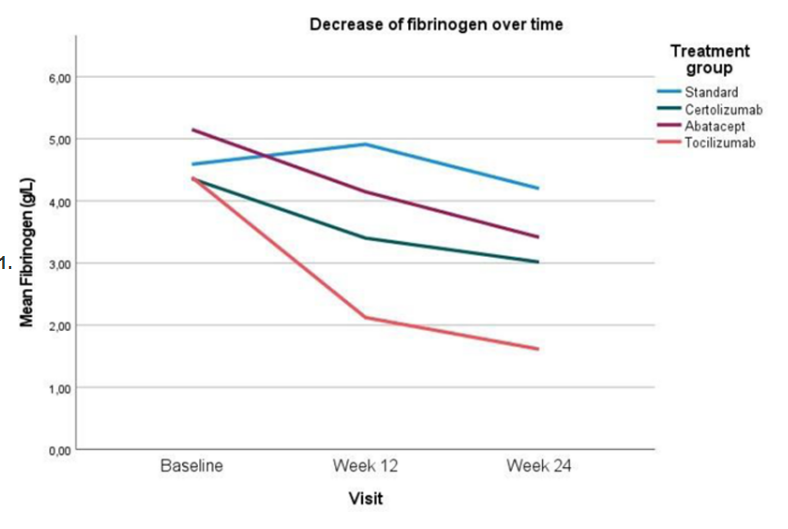

Aurelie Najm AurelieRheumo
3 years 7 months ago
NORD-STAR TRIAL drug does better in ttmt naive eRA? 48wks
CDAI remission ▶️ w/ ABA and CERTO PEG vs. MTX+ GCs while TCZ not stat ▶️.
Is the difference clinically sufficient to recommend these in first intention over MTX ? not convinced 🧐
OP0058 #EULAR22
@rheumnow

Richard Conway RichardPAConway
3 years 7 months ago
Damien et al on Whipple's Disease presenting as RMD. 73 cases (wow!) Treat Whipples, 93% remission, 94% off DMARD @RheumNow #EULAR2022 OP0066 https://t.co/XQnQq4RNcq

Robert B Chao, MD doctorRBC
3 years 7 months ago
10 year study showed NSAIDs associated with slowing radiographic progression in axSpA patients. mSASSS progression numerically lower in COX2i pts compared to non-selective NSAIDs.
@RheumNow #EULAR2022 #ABSTOP0021 https://t.co/dfz3rseikl
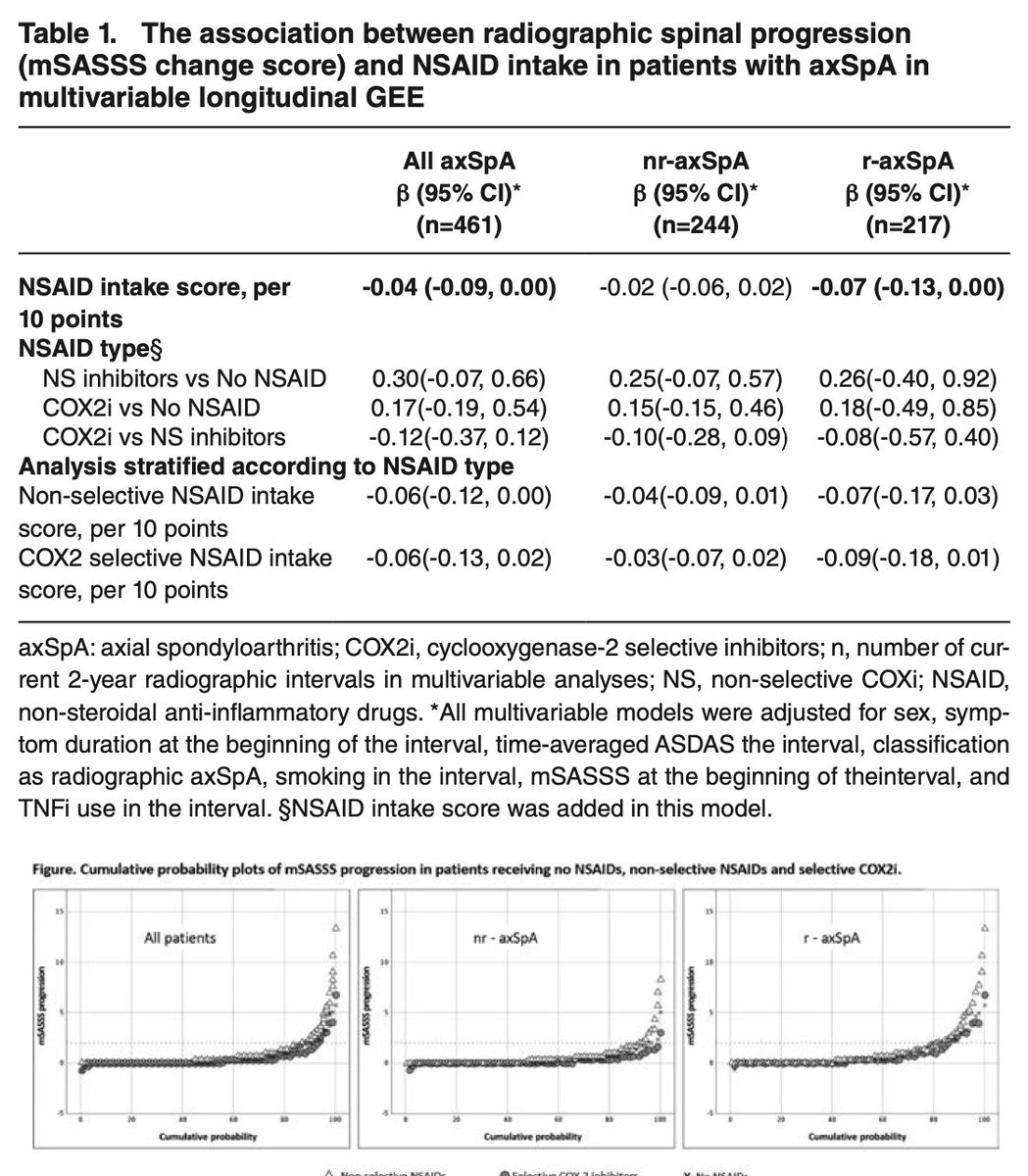

Robert B Chao, MD doctorRBC
3 years 7 months ago
Combination tx of NSAIDs and TNFi (celecoxib and golimumab) did not show significant superiority compared to TNFi alone in slowing radiographic progression for treatment of radiographic axSpA. There was a numerical reduction however.
#EULAR2022 @RheumNow #ABSTOP0018 https://t.co/gUZAwd92dK
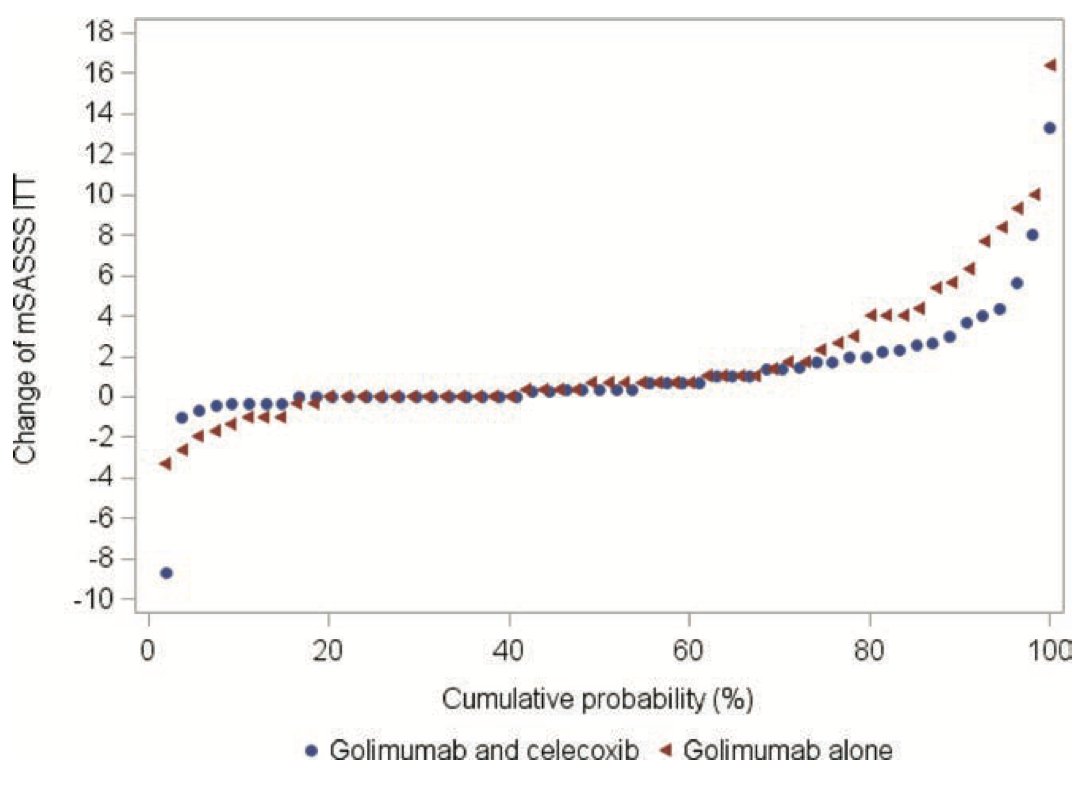

Eric Dein ericdeinmd
3 years 7 months ago
@Rheumnow #EULAR2022 POS0240
▶️ In 2021, 73.6% of Belgian RA patients staring a JAKi, started a 2nd generation JAKi (UPA, FIL).
Predictors for 1st generation usage (BAR, TOFA): older age, prior DMARD therapies.
What would you start as a first JAKi?

Eric Dein ericdeinmd
3 years 7 months ago
ACR21: conditionally recommends against steroids with starting csDMARD
#EULAR2022 recommends with rapid GC dose reduction and discontinuation
Which is better recommendation?
@RheumNow

TheDaoIndex KDAO2011
3 years 7 months ago
#EULAR2022 RA updated reccs w/ minor changes: start MTX+GC, reduce GC rapidly, JAKi are recc only for pts w/o risk factors for CV or malignant dz (not sure I agree with this last one if the JAKi is the best drug for the pt and there are little options avail) @rheumnow https://t.co/CXyyCi6LVI










 Poster Hall
Poster Hall