Novel Rx

Dr. John Cush RheumNow
3 years 5 months ago
534 PsA & 470 AS pts (SERENA study) on Secukinumab for avg of ~88wks - 5.8% PsA & 8.9% AS pts had sudden stop of SEC (for avg ~25wks) (reasons? Mostly AE 58% or pt decision 10%). While TJC & SJC increased, dz control returned w/ restarting SEC https://t.co/GFxj3b06Ra https://t.co/B2ubakq8Ql


Dr. John Cush RheumNow
3 years 5 months ago
The 2022 ACR abstracts are published and posted for you. Let the Learning begin! https://t.co/ZqMAc8jV3U https://t.co/3iCGnzsPI5

Deucravacitinib (Sotyktu), a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, is the only approved TYK2 inhibitor worldwide and the first innovation in oral treatment for moderate-to-severe plaque psoriasis in nearly 10 years.

Dr. John Cush RheumNow
3 years 5 months ago
FDA has approved SPEVIGO® (spesolimab-sbzo) an IV injection - the first-approved treatment option for generalized pustular psoriasis (GPP) FLARES. Spesolimab is an interleukin-36 receptor antagonist. https://t.co/zmuB1tyw9W https://t.co/bJiQB5qJNl

Can we predict the bad outcomes? Like when ITP evolves into SLE; or when psoriasis will develop arthritis; or if Sjogren's will develop lymphoma? Let's dive in and review these journal reports and this past week's news from RheumNow.com.

Dr. John Cush RheumNow
3 years 5 months ago
Rheums! Have a Rheumatology question or case for Jack Cush? Record it here and we may feature it on an upcoming podcast. Tell us your name and where you practice rheumatology.
https://t.co/1aSrRsLkel https://t.co/gmh155ty6w


Dr. John Cush RheumNow
3 years 5 months ago
STARTER study looked at US in 256 RA pts completed the study & were Rx w/ biologics csDMARDS or combination DMARDs. Pts w/ US grey scal or power doppler tenosynovitis better controlled in combination DMARDs than csDMARDs alone (p:0.025) https://t.co/MXLVoIxzmw https://t.co/8N5aV3vAA0

Dr. John Cush RheumNow
3 years 5 months ago
Nintedinib has been studied in 39 children (6–17 yrs) with fibrosing ILD demonstrating pharmacokinetics (wt based dosing) and safety. Adjusted mean changes in FVC % at week 24 were 0.3 with nintedanib and −0.9 with placebo https://t.co/yQ7OREzpTR https://t.co/iylG9BZiz4


Dr. John Cush RheumNow
3 years 5 months ago
FDA has approved SPEVIGO® (spesolimab-sbzo) an IV injection - the first-approved treatment option for generalized pustular psoriasis (GPP) FLARES. Spesolimab is an interleukin-36 receptor antagonist. https://t.co/izmExVCvfQ https://t.co/FELKR70UMv

Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.com.
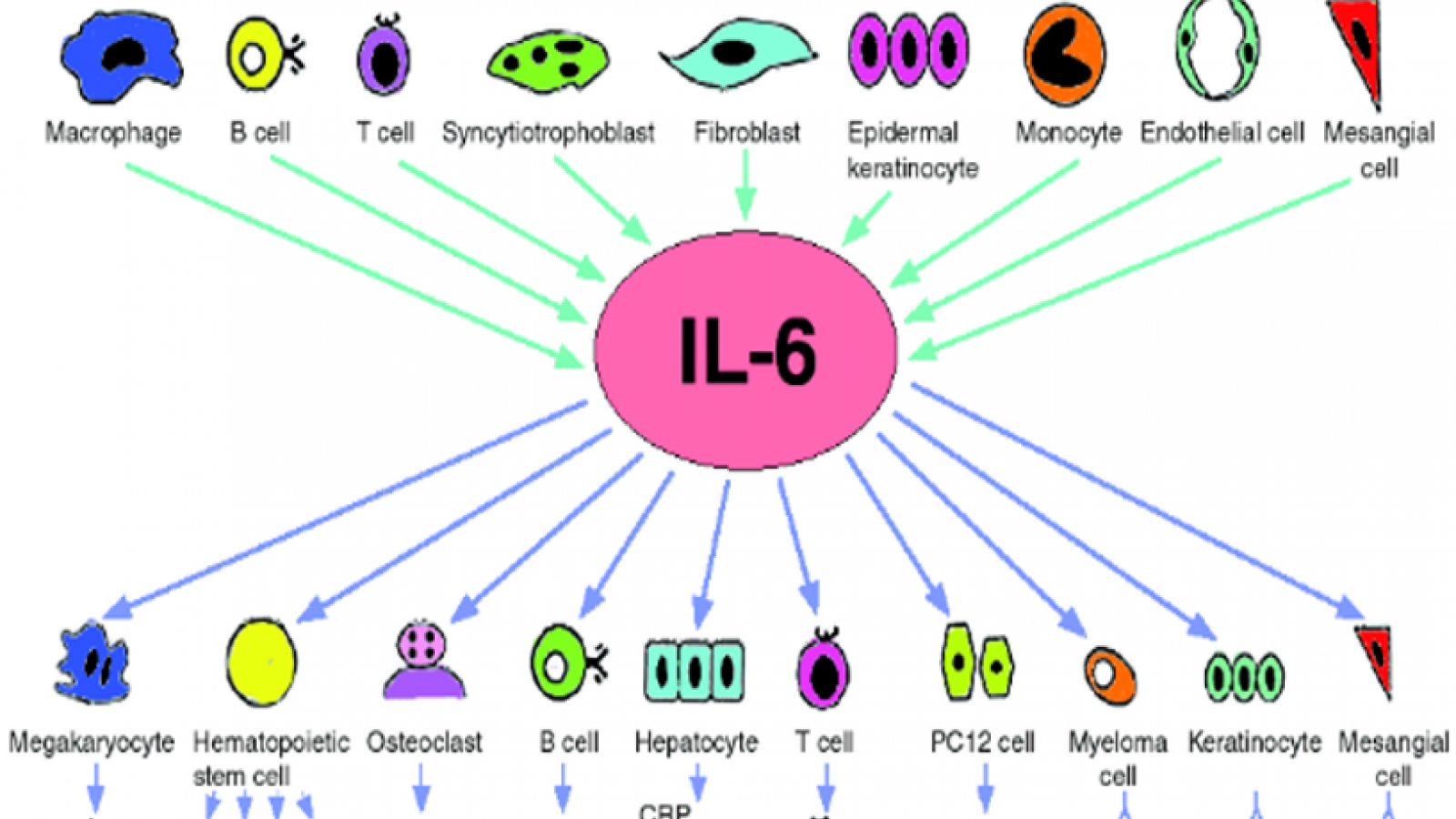
This week's NEJM has published the efficacy results of a large phase 3 trial of olokizumab, a humanized monoclonal antibody that directly targets IL-6 in patients with rheumatoid arthritis.
This is in contrast to two other marketed IL-6 inhibitors (sarilumab, tocilizumab) that bind to the IL-6 receptor.

Dr. John Cush RheumNow
3 years 5 months ago
Rituximab Efficacy in Systemic Sclerosis
The DESIRES trial studied rituximab (RTX) in patients with systemic sclerosis (SSc) and showed clinically significant improvement in skin and lung outcomes after a subsequent 24-week open-label extension phase.
https://t.co/8wWrt8GQ5v https://t.co/CIQKUyngN5
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow and discusses a case of refractory juvenile dermatomyositis with calcinosis.
The DESIRES trial studied rituximab (RTX) in patients with systemic sclerosis (SSc) and showed clinically significant improvement in skin and lung outcomes after a subsequent 24-week open-label extension phase.

Dr. John Cush RheumNow
3 years 6 months ago
Rheums! Do you have a rheumatology question or case for Jack Cush? Record it here and we may feature it on an upcoming podcast. Tell us your name and where you practice rheumatology.
https://t.co/cvmbzknQqA https://t.co/6mX5eRUNuU












 Poster Hall
Poster Hall