Drug Safety

David Liew drdavidliew
1 year 7 months ago
I think if we’re worried about JAK inhibitor safety, we need to be doing it right.
I wrote this for @RheumNow for #EULAR2024 ahead of my debate against @Janetbirdope, but also drew my inspiration from @RADoctor in @ACR_Journals - a must read:
https://t.co/RA6ktQAzqO https://t.co/lltDbtxpAg

Peter Nash drpnash
1 year 7 months ago
important session worth reading the full recommendations - TNF tick of approval @RheumNow #EULAR2024 https://t.co/ORDocVie11


Md Yuzaiful Md Yusof Yuz6Yusof
1 year 7 months ago
#EULAR2024 Please find my short blog on the Impact of Safety Warning Update on the use of JAK-inhibitors @RheumNow
https://t.co/Z4pujQRQ9h https://t.co/QRUnJInblH


David Liew drdavidliew
1 year 7 months ago
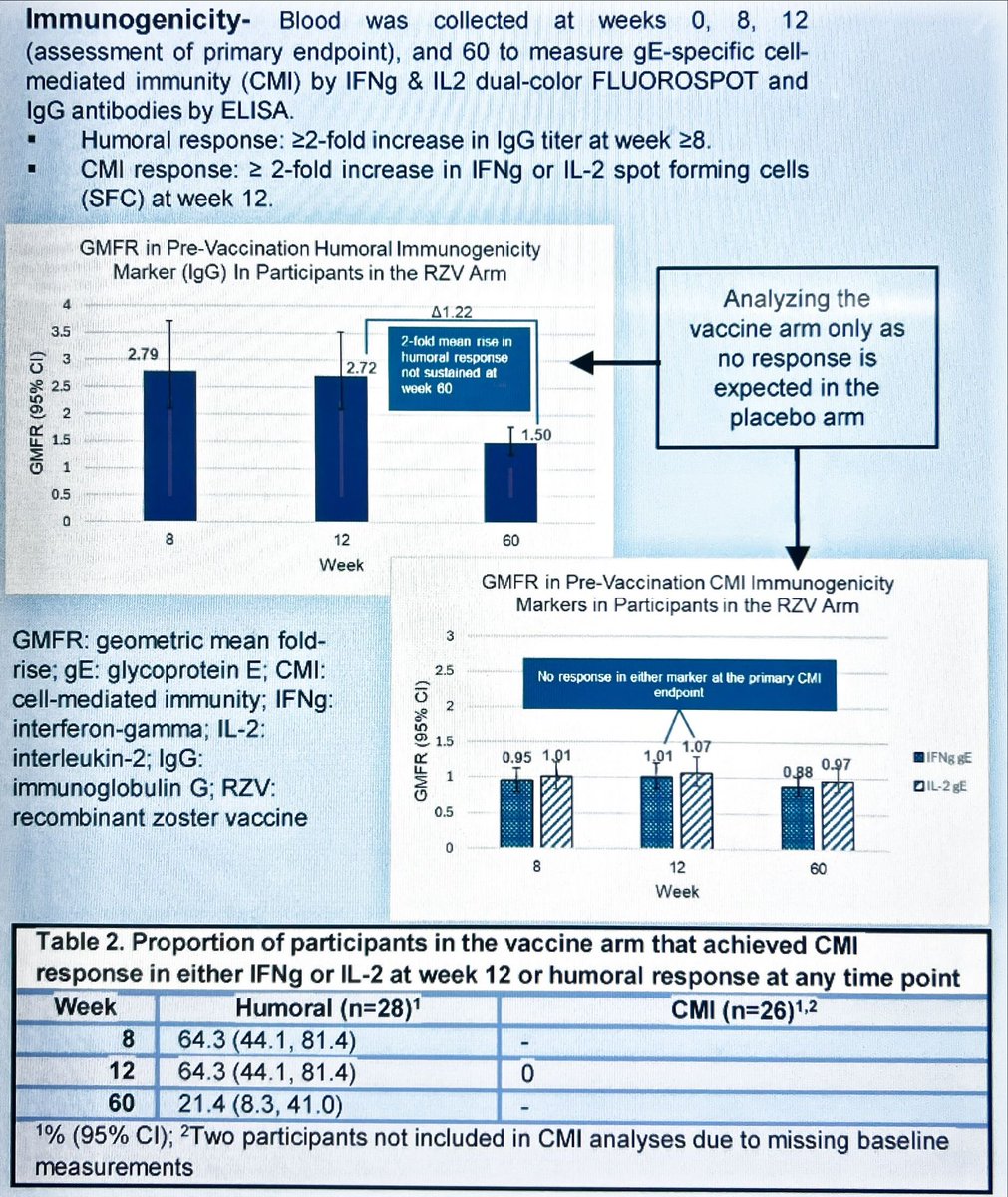
RA patients on abatacept have an appalling immunogenic response to Shingrix. Humoral fades away in the first year, and an absence of cellular responses.
Alarming and need strategies to combat this #EULAR2024 POS0620 Winthrop/@RADoctor @RheumNow https://t.co/8gY9ocLBDp

Md Yuzaiful Md Yusof Yuz6Yusof
1 year 7 months ago
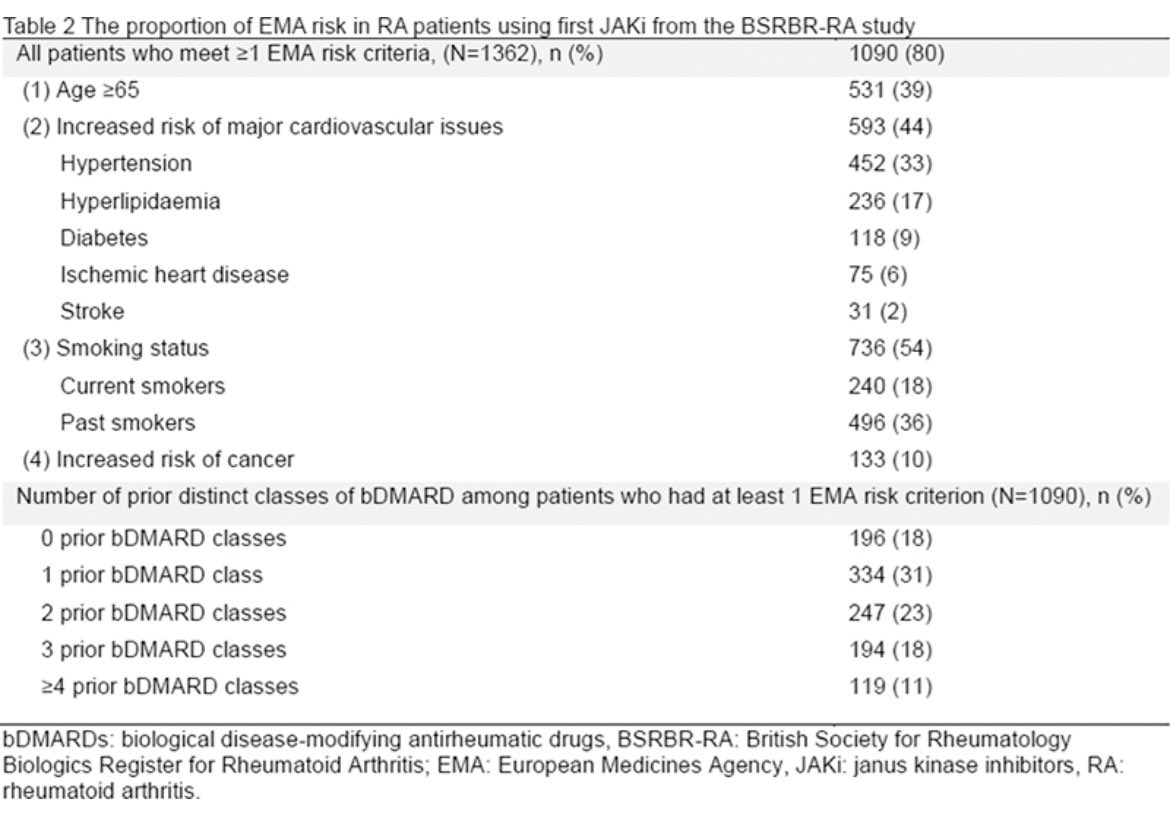
#EULAR2024 POS0390 What impact of regulatory warning on JAK-i on access to therapy? Data from UK BSSR-BR reported 4 in 5 #RA patients would have been considered ‘high-risk’. Of these, 28% had failed ≥3 biologics; so no suitable alternatives. Enormously impacted @RheumNow https://t.co/XeS9qHXSpH

Dr. Antoni Chan synovialjoints
1 year 7 months ago
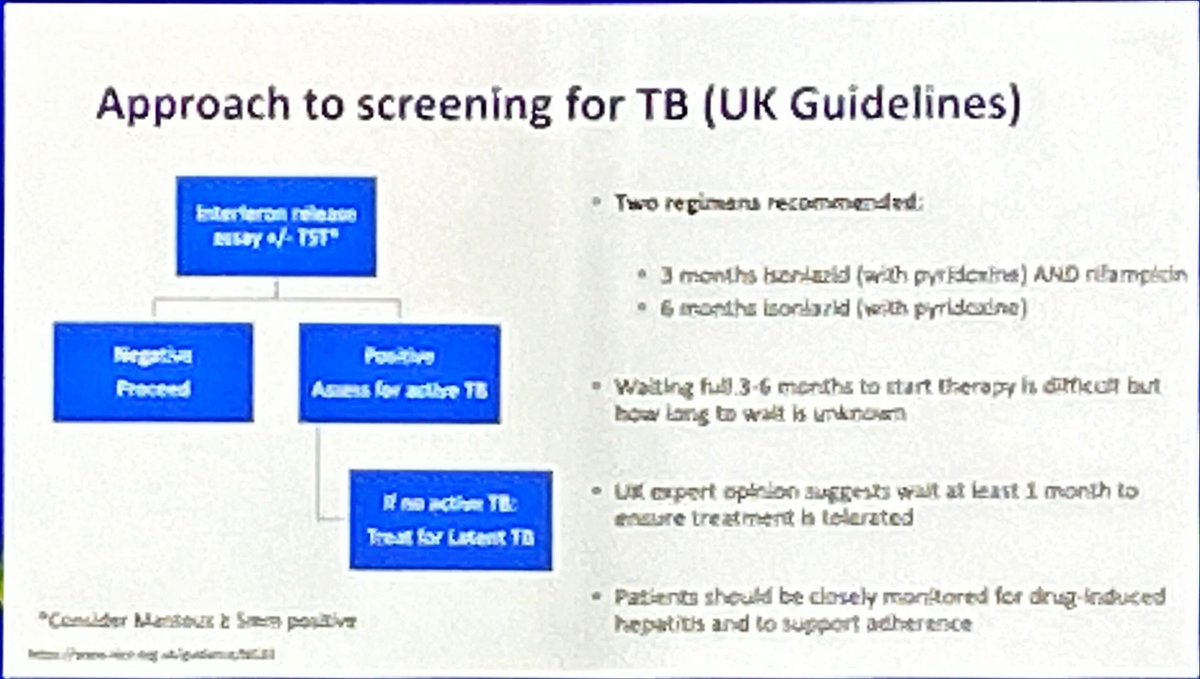
Approach to TB screening prior
to commencing biologics. Hyrich K #EULAR2024 @RheumNow https://t.co/j3PI0PHpqW
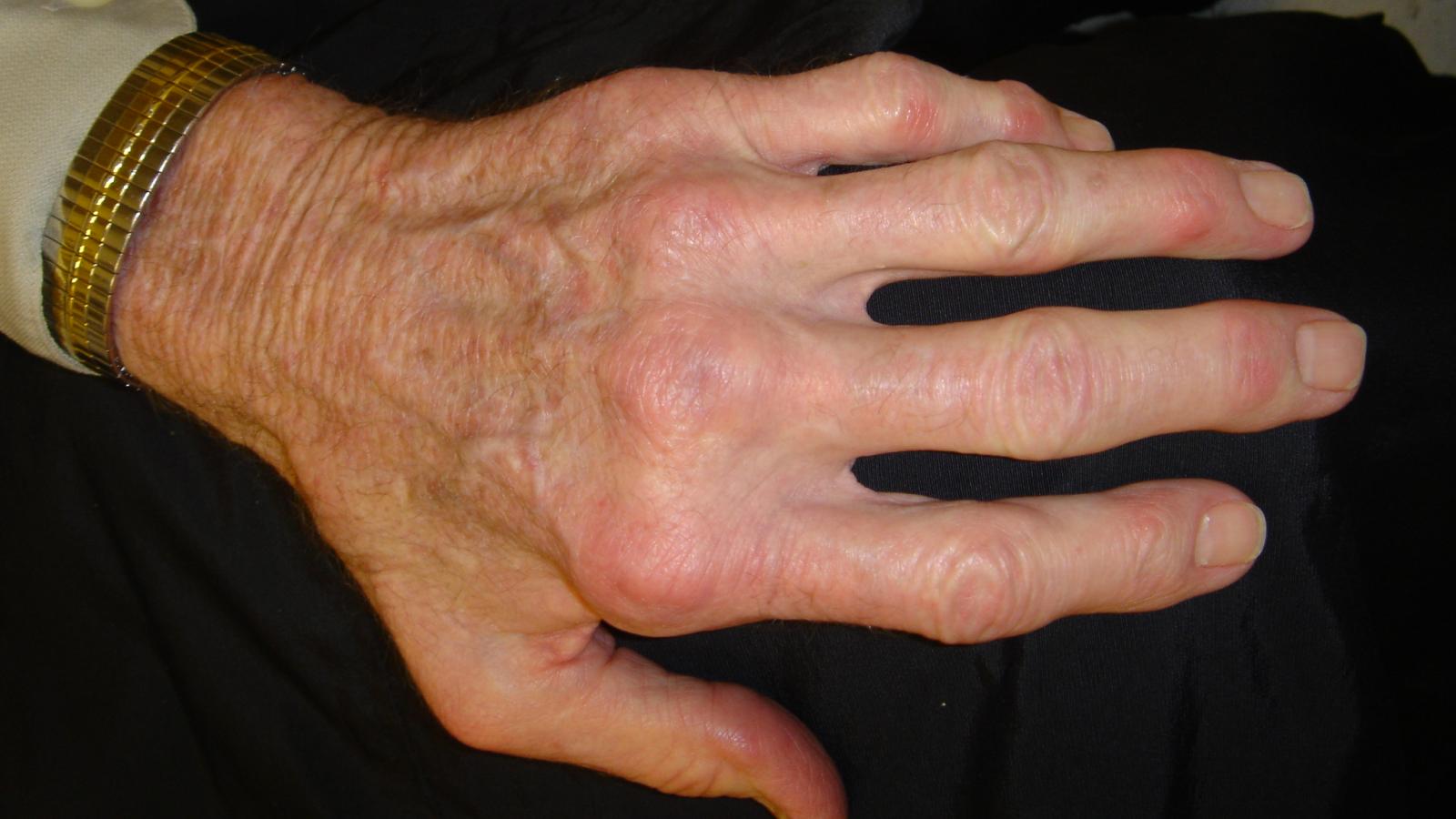
Multi-modal analysis of rheumatoid arthritis (RA) synovitis pathotypes enables more accurate choices of first line cDMARDs treatment, suggesting a predictive value to synovial biopsy.

Dr. John Cush RheumNow
1 year 7 months ago
The art of talking about risks with our patients
https://t.co/V0JQ1EsbKc https://t.co/GSTbPu0EXZ


Md Yuzaiful Md Yusof Yuz6Yusof
1 year 7 months ago
#EULAR2024 Please find our Day 1 & Day 2 Recap of selective abstracts/research presented at the conference 😃@RheumNow @DrMiniDey
https://t.co/AHl18gS7gF https://t.co/LEoa9rBNly


Dr. Antoni Chan synovialjoints
1 year 7 months ago
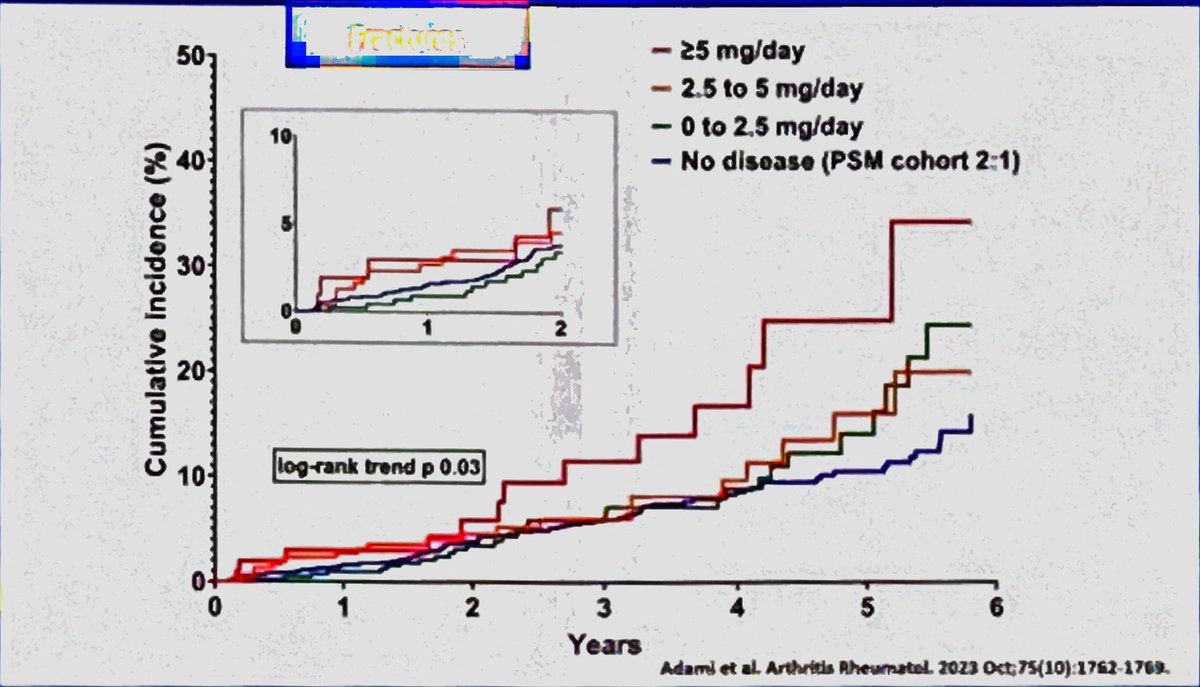
The fracture risk with glucocorticoids is dose dependent. Lems W #EULAR2024 @RheumNow https://t.co/TGjKu1vfXx
Day 2 at EULAR 2024 was a big poster day for many with several good sessions and oral presentations on Preventing RA, new vasculitis therapies and a session devoted to the 50th anniversary of the Moll & Wright Criteria.

Dr. John Cush RheumNow
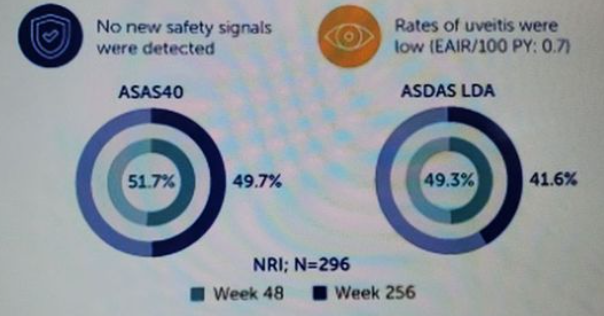
1 year 7 months ago
Long term safety of bimekizumab (BKZ) in ankylosing spondylitis (BE AGILE) study (n 296) shows sustained ASAS40 efficacy @wk 48 (52%) & wk256 (50%). Low AE for candidiasis (2.6%), SIE (1.4%), IBD (0.8/100PY), Uveitis (0.7/100PY) Abst# POS0215 #EULAR2024 https://t.co/9PWey3itu9

David Liew drdavidliew
1 year 7 months ago
I was allocated to bring down the warning, & I did apologise up front to both the EMA and Janet #EULAR2024. It's a tough gig regulating impactful medicines with risk-benefit, especially when our world is full of other medicines with risk-benefit trade-offs (like prednisolone). 2/ https://t.co/6YPhYHbhg8










 Poster Hall
Poster Hall