Lupus

1 year 10 months ago
#EULAR2022 LB0005
Orelabrutinib- BTKi- for SLE, PIb/IIa
⭐️Improved SRI-4 and SLEDAI-2K vs PBO, dose response for SRI-4
⭐️Improved proteinuria
@Rheumnow https://t.co/bmREhmL7sq

1 year 10 months ago
Another possible Rx for #SLE:
Phase Ib/IIa orelabrutinib (irrevers BTKi) for SLE shows promise. Improvement in SRI-4, SLEDAI2K, proteinuria, dsDNA, C3/C4; dec in IgG/M/Bcells. Safety: lymphopenia w/ORE #EULAR2022 LB0005 @rheumnow https://t.co/etZ9hMYNfn

1 year 10 months ago
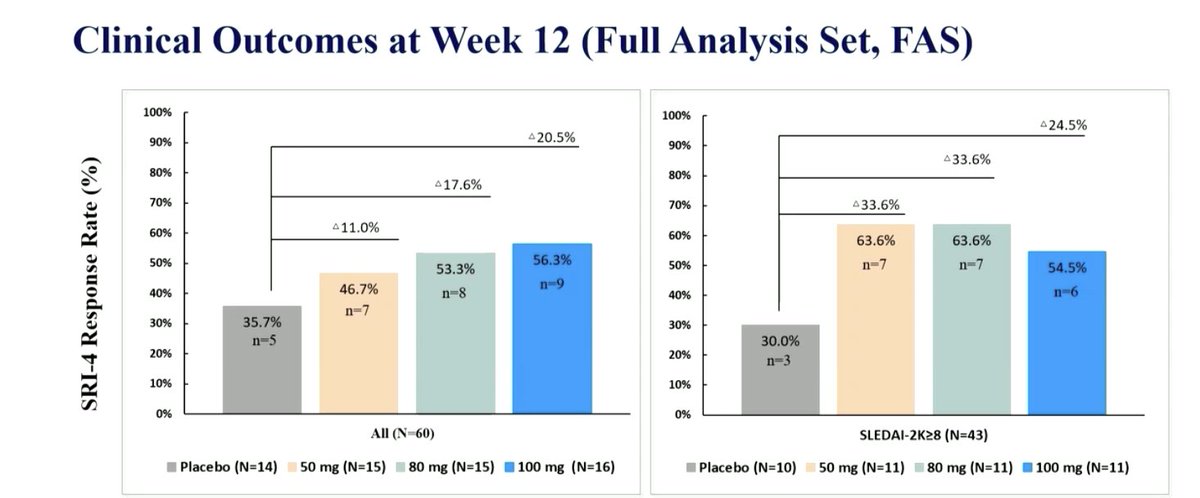
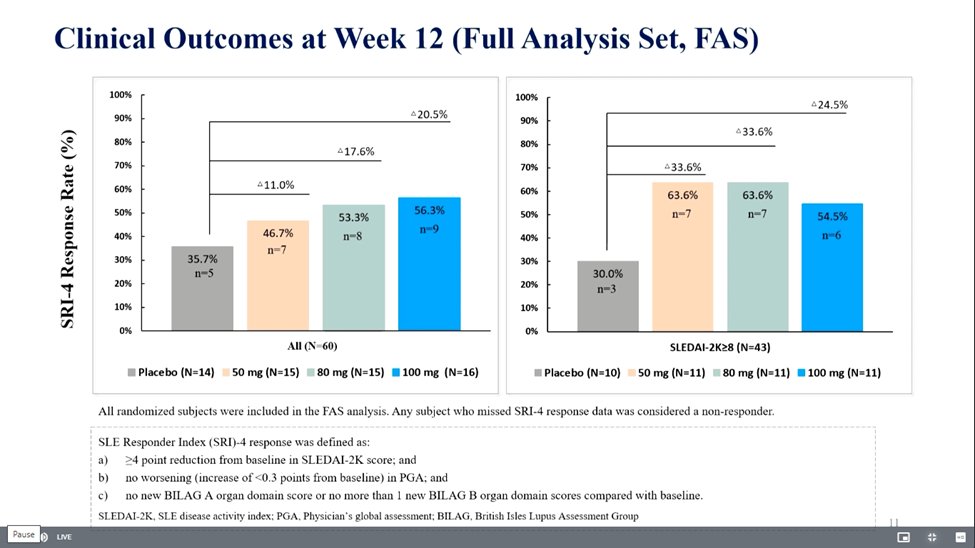
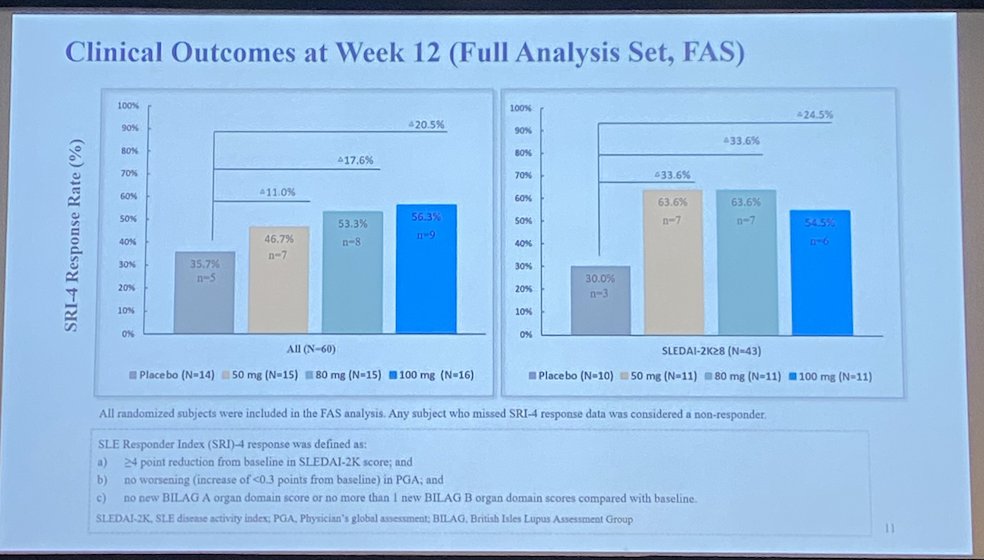
Orelabrutinib Bruton’s
tyrosine kinase inhibitor in SLE
RCT phase Ib/IIa
SRI(4) wk 12 50mg 50% 80mg 61% and 100mg 64% and 37.5% PBO
Trend ⬇️ proteinuria, anti-dsDNA & IgG
Safety: 3SAEs in OLE group
LB0005 @RheumNow #EULAR2022 https://t.co/4r16NTaZvT

1 year 10 months ago
@EricFMorand up first in late-breakings
PAISLEY: deucravacitinib (TYK2i) ph2 in SLE - add-on, steroid wean
Good outcomes:
primary: SRI(4) at 32w
skin/joint/LLDAS & dsDNA/C4
Safety good (like PsO/PsA) but higher dose less so
Watch for: other clinical, ph3
#EULAR2022 @RheumNow https://t.co/XOQWuiHkTx

1 year 10 months ago
Wow! Phase 2 #deucravacitinib data for #SLE met primary & secondary endpoints: SRI(4) response, BICLA, LLDAS, CLASI-50. AEs include: skin related events and UTIs but no increase in SIE, HZ, MACE/VTE @bmsnews LB0004 #EULAR022 @rheumnow https://t.co/bsKI0mixVT

1 year 10 months ago
#POS1236 #EULAR2022 More data to justify safety of #COVID vaccination to our #lupus patients. A study in Italy (>450 pts) found 1/4 had side effects (mostly mild) and 4% had a flare. These side effects were more common in those on >1 DMARDS and Belimumab @RheumNow https://t.co/mNS6rQfsSq
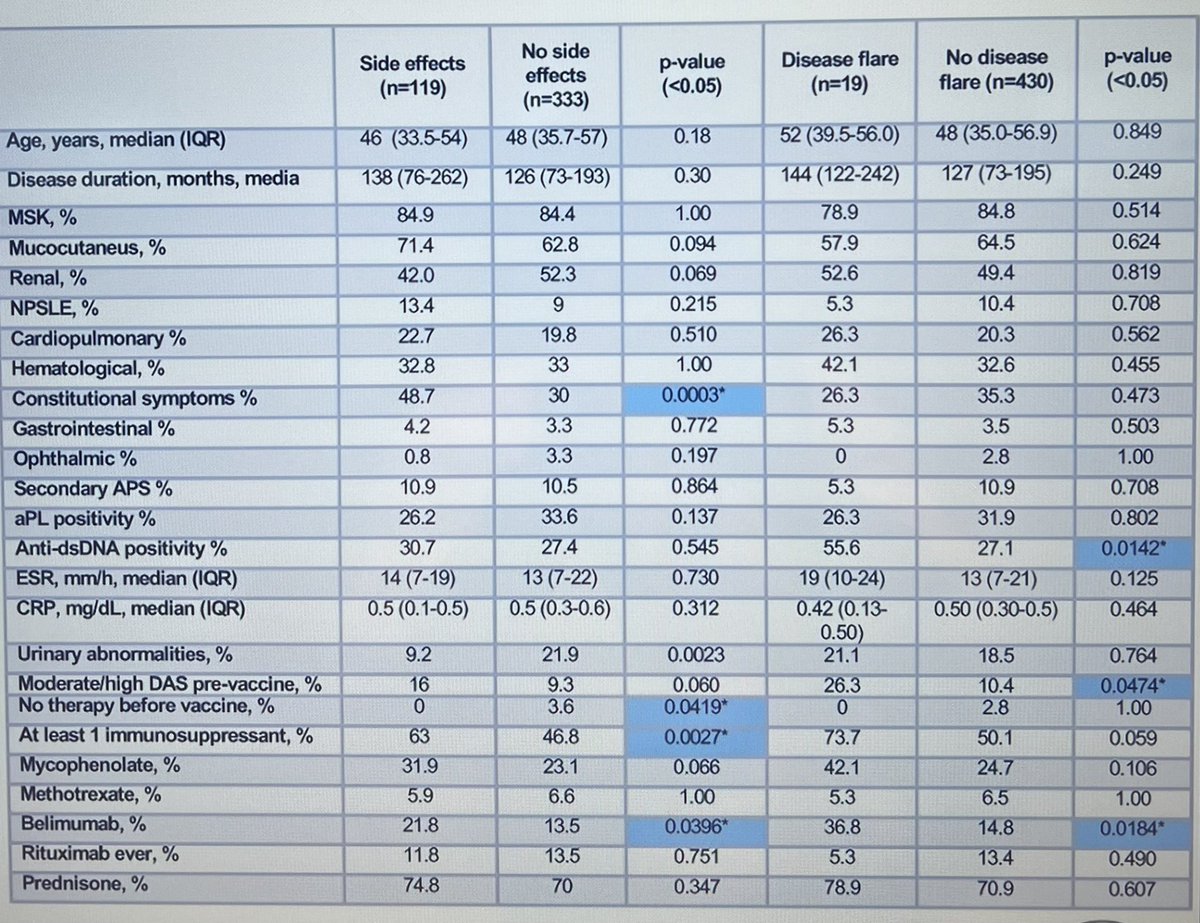

1 year 10 months ago
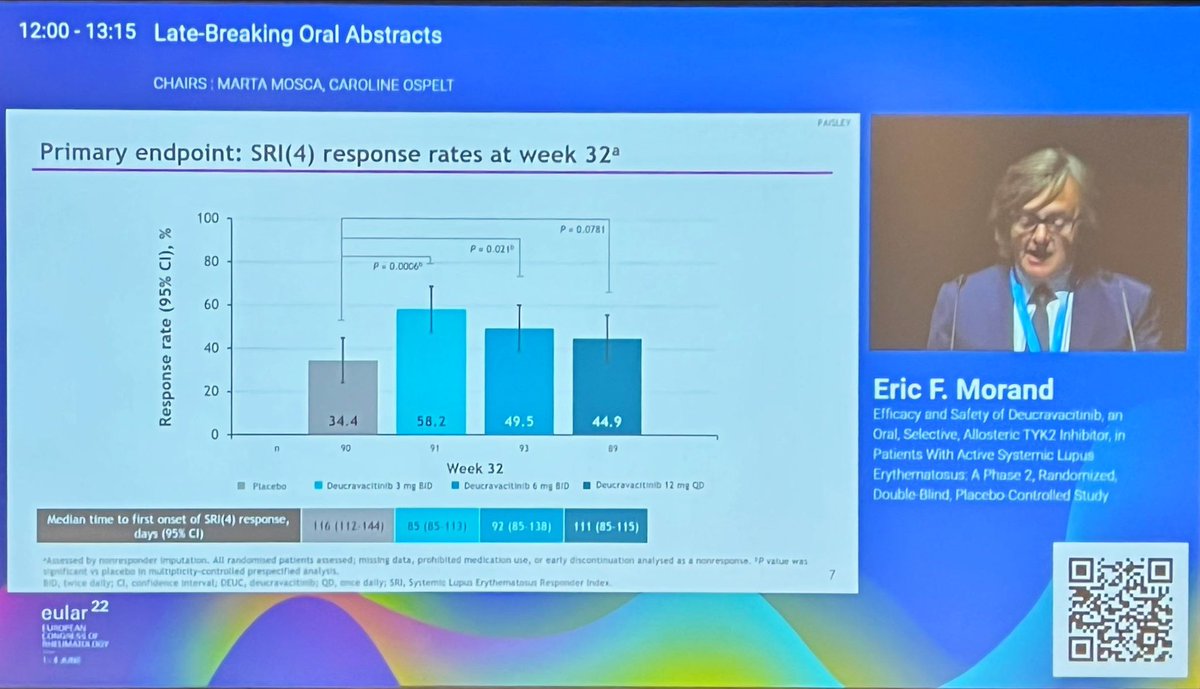
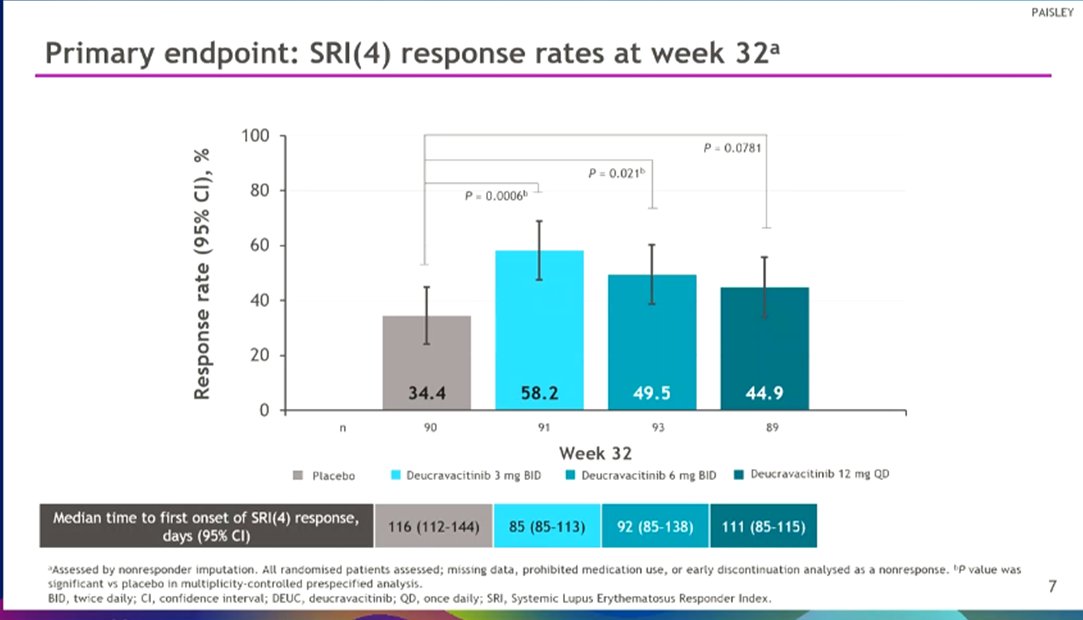
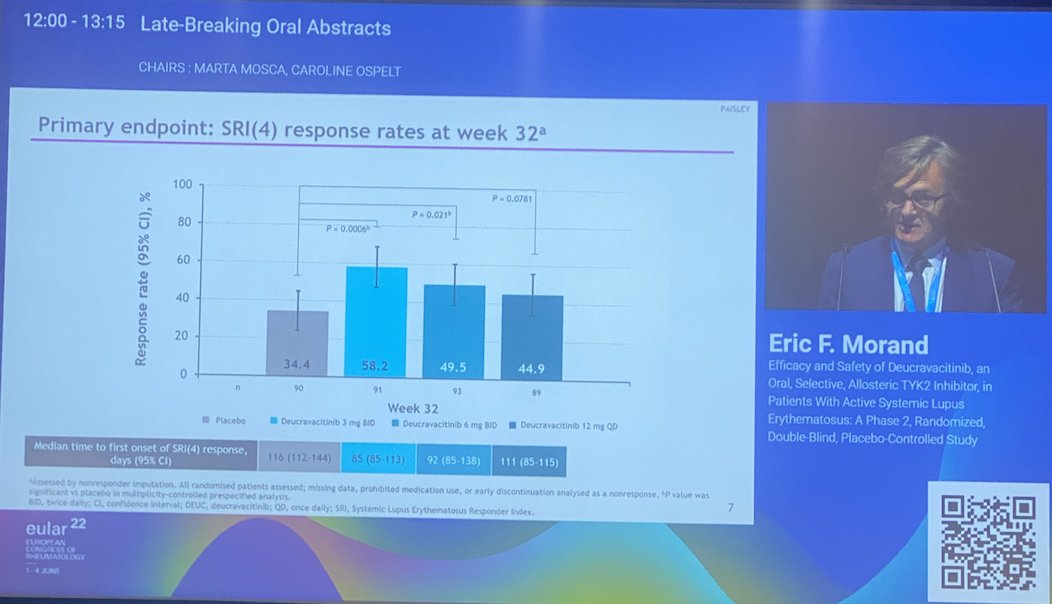
Morand et al Tyk2i deucravacitinib in SLE. 48-week phase 2 RCT. SRI(4) PBO: 34.4%; DEUC 3 mg BID: 58.2%, P =0.0006; DEUC 6 mg BID: 49.5%, P =0.021; DEUC 12 mg QD: 44.9%, P =0.078. BICLA, LLDAS, CLASI-50, active joint count also+ @RheumNow #EULAR2022 LB0004 https://t.co/MBPc9xQFvG https://t.co/n3GUwl0RtD

1 year 10 months ago
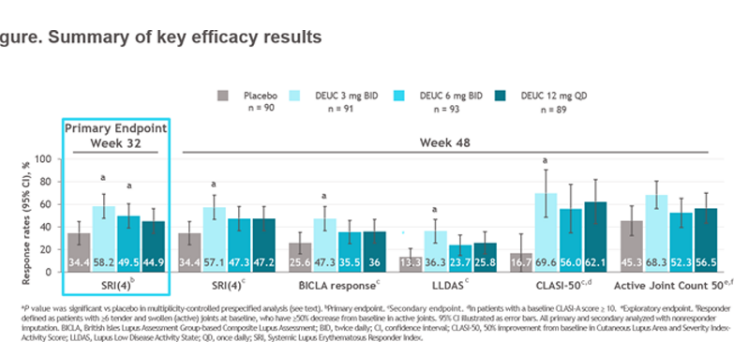
PAISLEY LB004
@EricFMorand on deucravacitinib phase 2 for SLE
⭐️Meets primary endpt: SRI(4)
⭐️Secondary endpts: BICLA, LLD, CLASI, jt count, biomarkers improved
⭐️Safety data wo VTE, CVD events
Earlier at #EULAR2022, BRAVE trial phase 3 baricitinib: No benefit in SLE
@RheumNow https://t.co/3YpPSJcenF

1 year 10 months ago
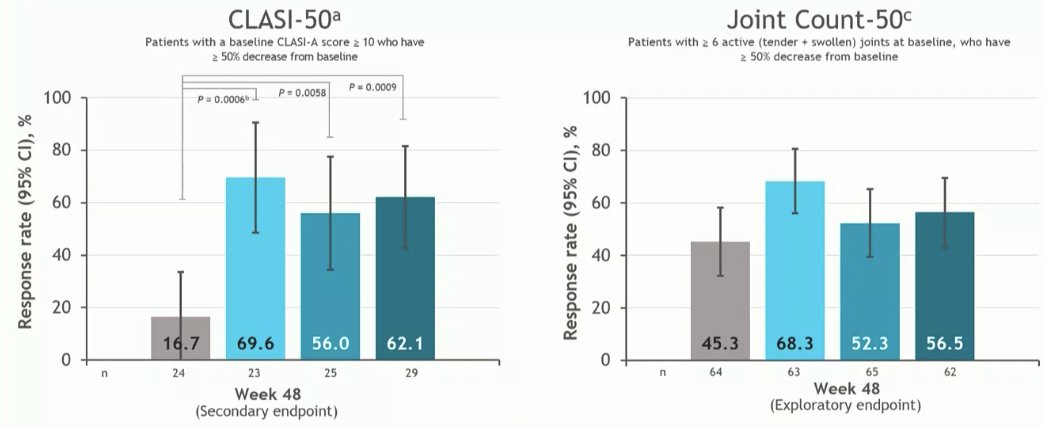
PAISLEY study Phase 2 RCT
Deucravacitinib in SLE meets primary endpoint wk 32
Results for dose 3mg BID:
*SRI(4) response 58.2% vs.
PBO 34%
*LLDAS 36%
*CLASI 70%
*⬇️ SJC
No new safety signal
Now waiting for Phase 3 👀
@RheumNow LB0004 #EULAR2022 #Lupus https://t.co/2KYSvfq4h7

1 year 10 months ago
Please see my video interview with Dr @LucyCarter6 on her prize winning #EULAR2022 abstract #OP0234 on transcriptomic analyses of peripheral bloods in identifying modules that predict OR protect progression from ANA+ to #lupus or CTD @RheumNow https://t.co/c3khwFzx79












 Poster Hall
Poster Hall