Novel Rx
This year at EULAR 2022, there were important and interesting topics in Axial Spondyloarthritis (AxSpA). These are my picks of abstracts from the conference.
For autoimmune patients with a history of malignancy, the initiation of biologic or targeted synthetic disease modifying agents (bDMARD/tsDMARDs) may provoke concern. While data for biologic medications and malignancy risk has been largely reassuring, clinical trials have often excluded patients with history of cancer.

Janet Pope Janetbirdope
3 years 7 months ago
#Bestinclass Recurrent #PMR RCT of #sarilumab v placebo. Nearly doubles sustained remission but maybe attenuating effect - flare difference over time? N=118. 🤷♀️ Dunno who I would use it in yet. LB0006 @RheumNow @eular_org #EULAR2022

Robert B Chao, MD doctorRBC
3 years 7 months ago
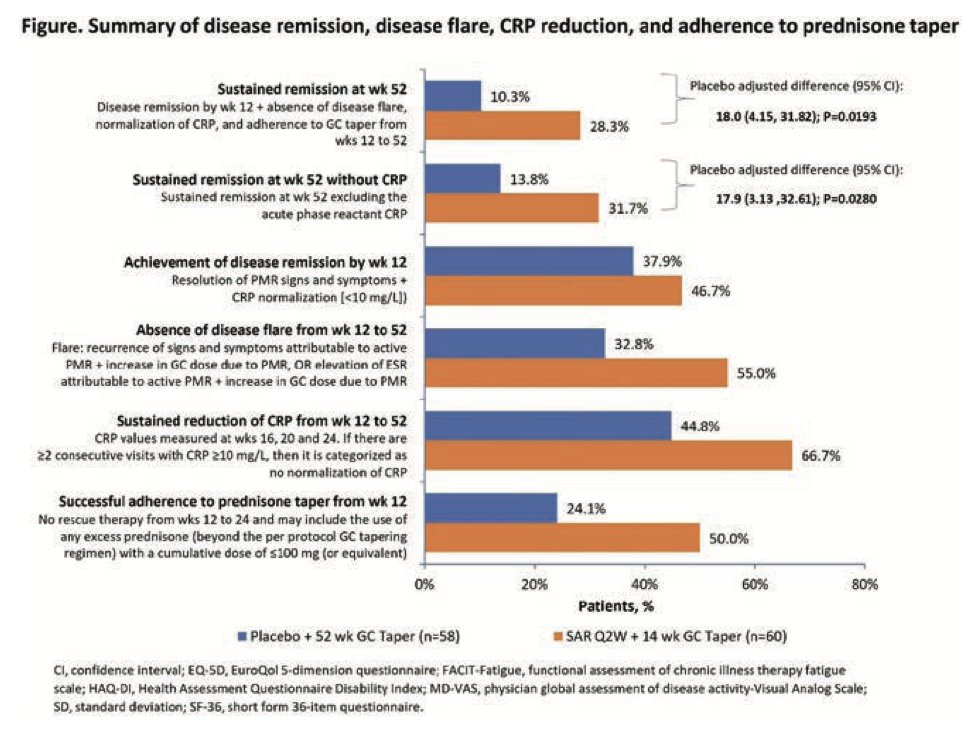
Sarilumab in pts with relapsing PMR - Phase 3 trial
Sarilumab +14 week steroid taper - higher sustained Remission than w/o sarilumab (28% vs 10%)
Less flare occurrences
Sarilumab noted to have higher incidences of neutropenia, arthralgia
@RheumNow #EULAR2022 ABST#LB0006 https://t.co/SeetkBsYI3

David Liew drdavidliew
3 years 7 months ago
Biosimilars and COVID vaccines have made nocebo more pertinent than ever.
How do we combat it?
First line is good communication, and the foot soldiers are the right phrases.
Language matters. #EULAR2022 @RheumNow https://t.co/U0Chv9ia1G


David Liew drdavidliew
3 years 7 months ago
Post-ORAL Surveillance, the @eular_org RA treatment guidelines had to change.
In the 2022 version:
JAKi now separate sentence following bDMARDs, with proviso to mention risk.
Sometimes guidelines have to tell us what we’ve all inevitably been expecting
#EULAR2022 @RheumNow https://t.co/RPbPNi3vcW


Md Yuzaiful Md Yusof Yuz6Yusof
3 years 7 months ago
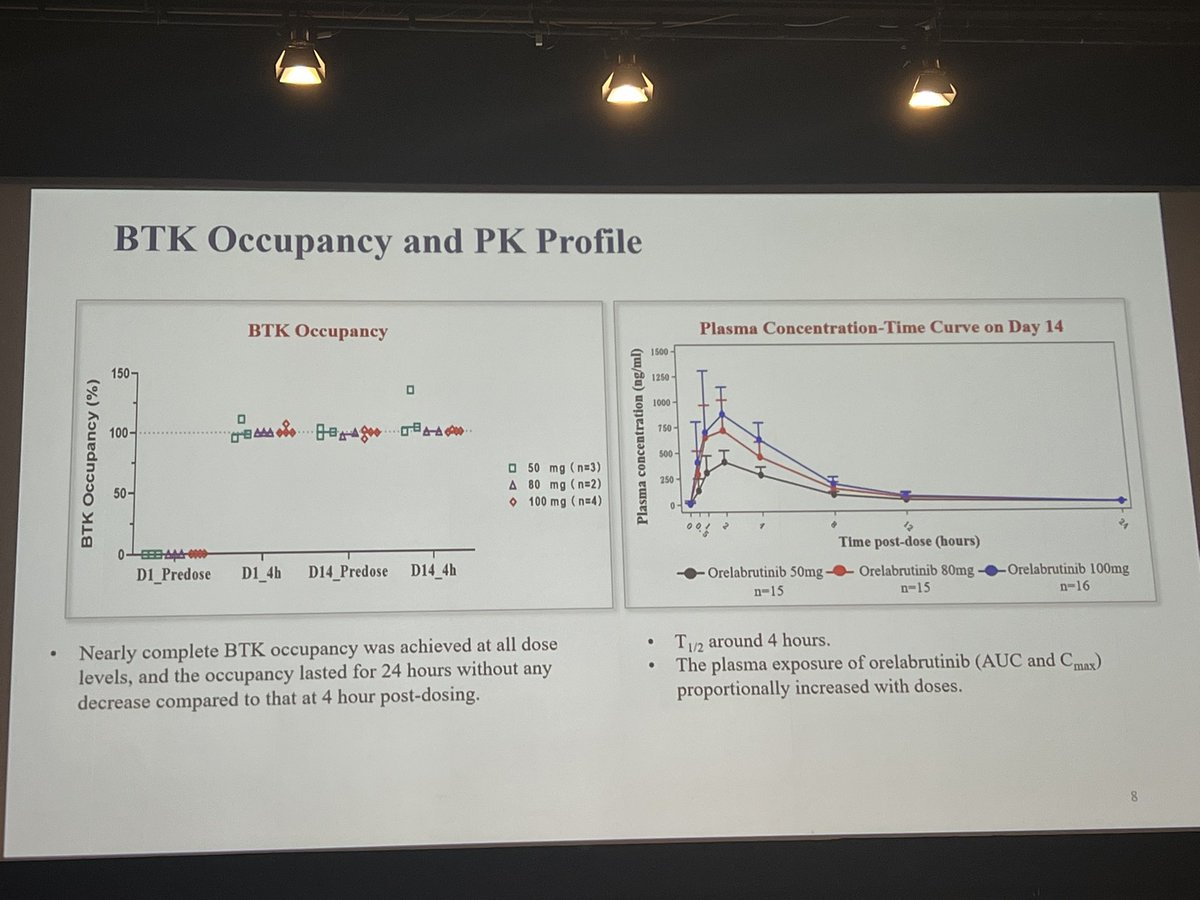
#LB0005 #EULAR2022 Phase 1B/2A dose-ranging RCT of Orelabrutinib, a BTK-inhibitor showed BTK occupancy in all doses in #lupus. SRI-4 response was higher in all doses vs PBO. Effects were better in SLEDAI=>8. Well tolerated & no major safety. Promising early data @RheumNow https://t.co/vJBLRQgYBP

Robert B Chao, MD doctorRBC
3 years 7 months ago
Does treating spondyloarthritis early matter?
Systematic review showed in nr-axSpA tx with biologics may lead to better outcomes compared to established axSpA
axSpA - no difference in response between early vs. established disease
@RheumNow #EULAR2022 ABST#POS0302

Aurelie Najm AurelieRheumo
3 years 7 months ago
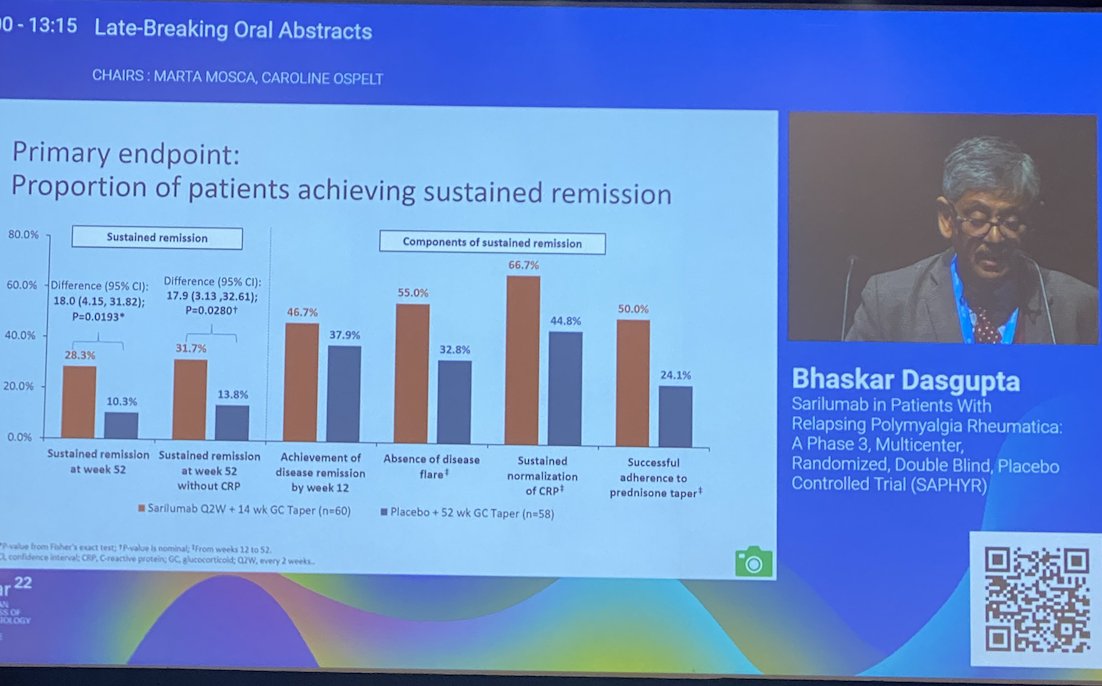
Sarilumab in PMR
SAPHYR Phase 3 RCT
Underpowered (<50% target recruitment)
-% remission sustained from wk12 to wk 52
28% vs 10% PBO
-Risk of flare reduced HR 0.56
-Improvement of several PROs
No new safety signal
LB0006 @RheumNow #EULAR2022 https://t.co/n7WdQxQaHo

TheDaoIndex KDAO2011
3 years 7 months ago
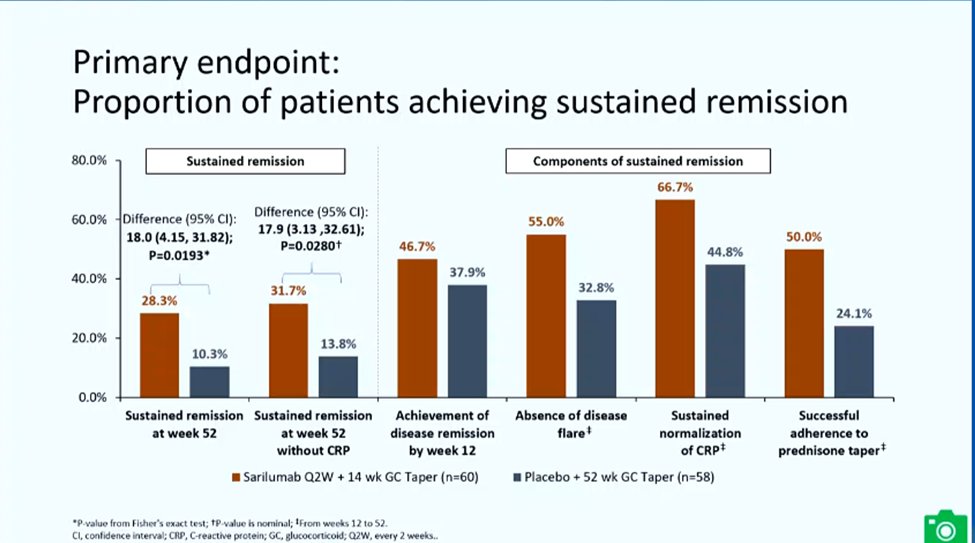
SAPHYR phase 3 RDBPCT study: #sarilumab vs PCB for relapsing #PMR on pred 7.5+ mg/d. Study met endpoints (whether of or not CRP was included). Time to first flare was longer w/SARI, less steroid use, and also better PROs. AE: #EULAR2022 LB0006 @rheumnow 1/2 https://t.co/lIspLKlveb

Richard Conway RichardPAConway
3 years 7 months ago
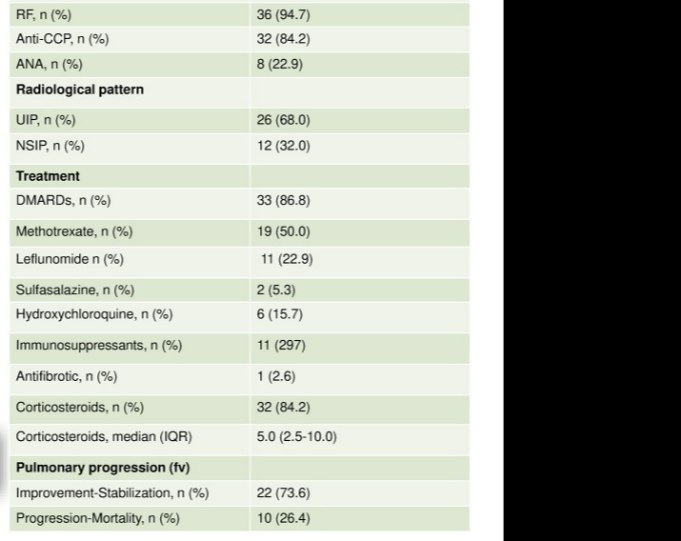
Redondo-Rodriguez et al. Abatacept in RA-ILD. Prospective study 38 patients. 73.6% improved/stablised with abatacept at median 17 month follow-up @RheumNow #EULAR2022 POS0654 https://t.co/D2du0hAXIy

Md Yuzaiful Md Yusof Yuz6Yusof
3 years 7 months ago
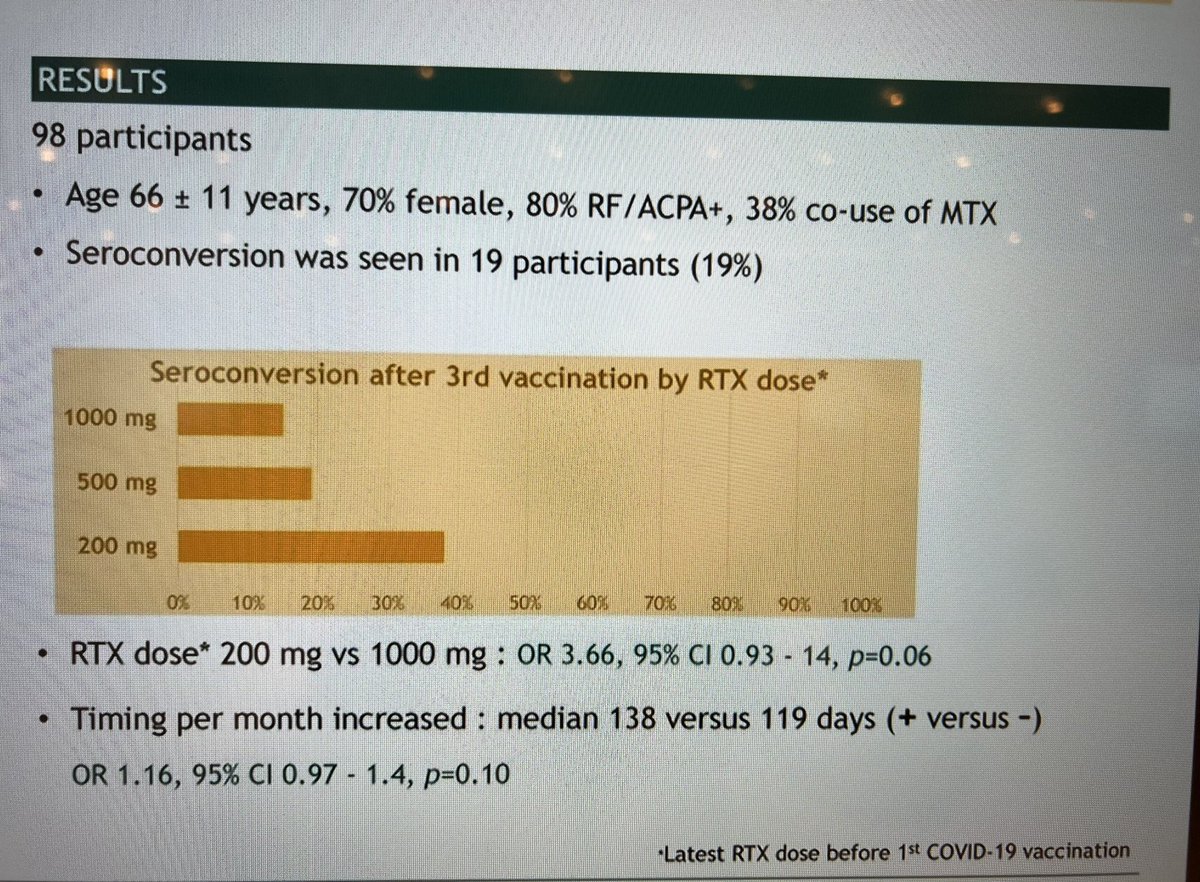
#POS1207 #EULAR2022 In N=98 RA patients with poor antibody response to rituximab after two doses, 19% responded after 3rd dose. Associated with longer Time of vaccine from last RTX & interestingly ultra dose 200mg used in the Netherlands @RheumNow https://t.co/TTXXtOU6vp

David Liew drdavidliew
3 years 7 months ago
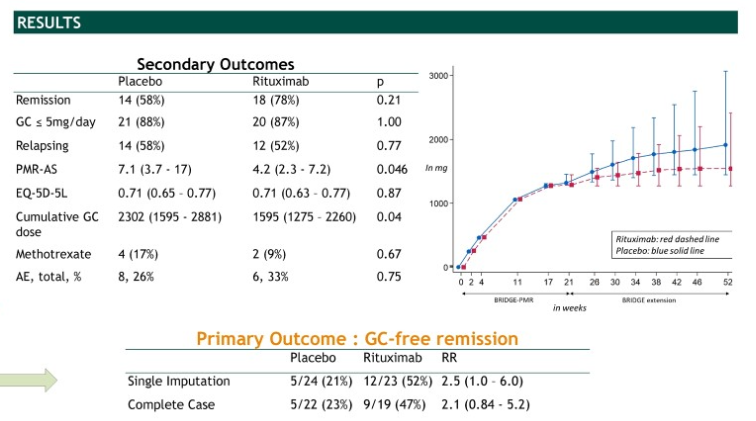
BRIDGE-PMR (rituximab in PMR) routine care extension to 52w
(in #PMRGCAJC one big qu asked was ?sustained benefit)
sustained benefit (RR 2.5 GC-free remission)
steroid-sparing (PNL 1.6g v 2.3g)
& ritux ?cheap enough now
but COVID calculus difficult
POS0269 #EULAR2022 @RheumNow https://t.co/Ln2ArclKO8 https://t.co/OtCwdjIPeL

Aurelie Najm AurelieRheumo
3 years 7 months ago
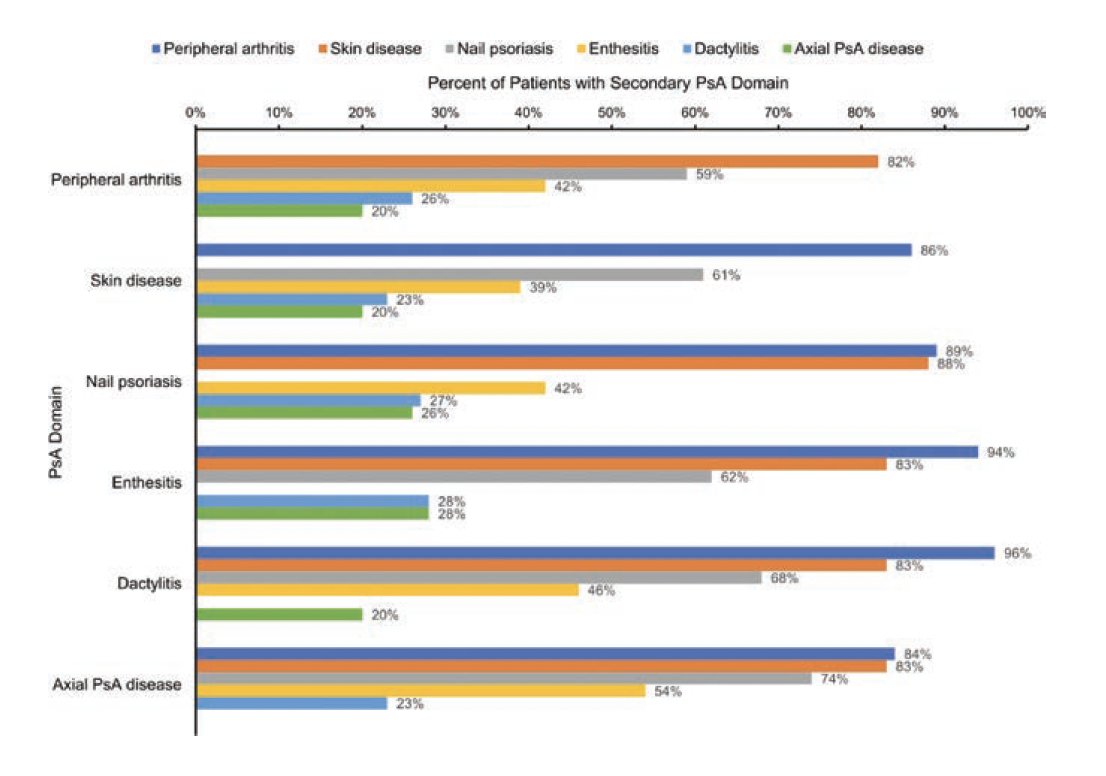
CorEvitas PsA Registry (1000+ pts)
Analysis of biologics prescription according to disease domains
No real surprise to see that IL-17i were prescribed more frequently in pts w/ PsO BSA>10% at BL
Overall first line TNFi 40% > IL-17i 14%
POS0309 @RheumNow #EULAR2022 https://t.co/dkLxl8d6ug











 Poster Hall
Poster Hall