JAK/TYK2

1 year 10 months ago
PAISLEY LB004
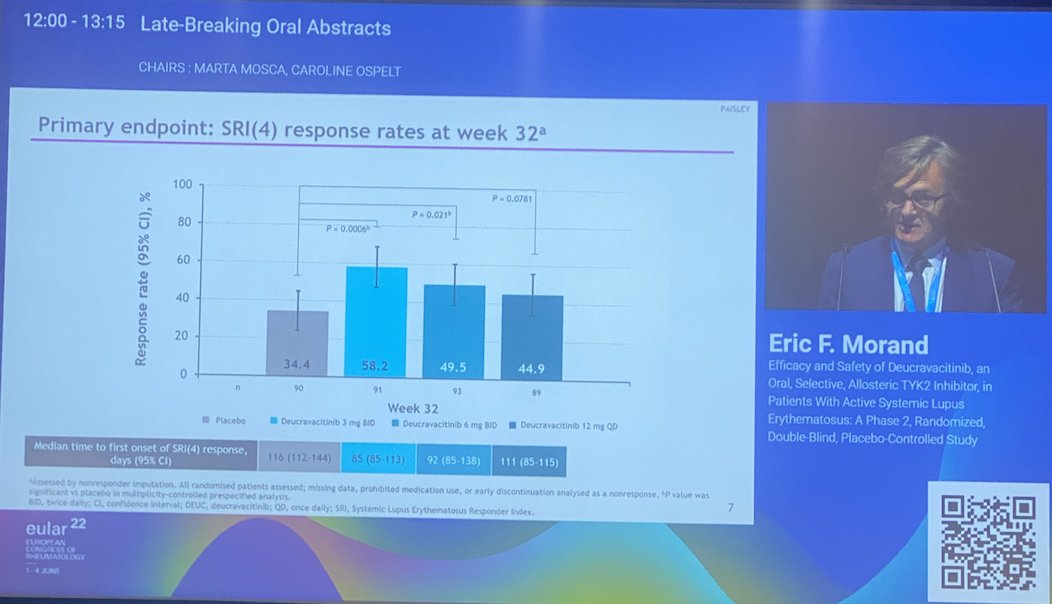
@EricFMorand on deucravacitinib phase 2 for SLE
⭐️Meets primary endpt: SRI(4)
⭐️Secondary endpts: BICLA, LLD, CLASI, jt count, biomarkers improved
⭐️Safety data wo VTE, CVD events
Earlier at #EULAR2022, BRAVE trial phase 3 baricitinib: No benefit in SLE
@RheumNow https://t.co/3YpPSJcenF

1 year 10 months ago
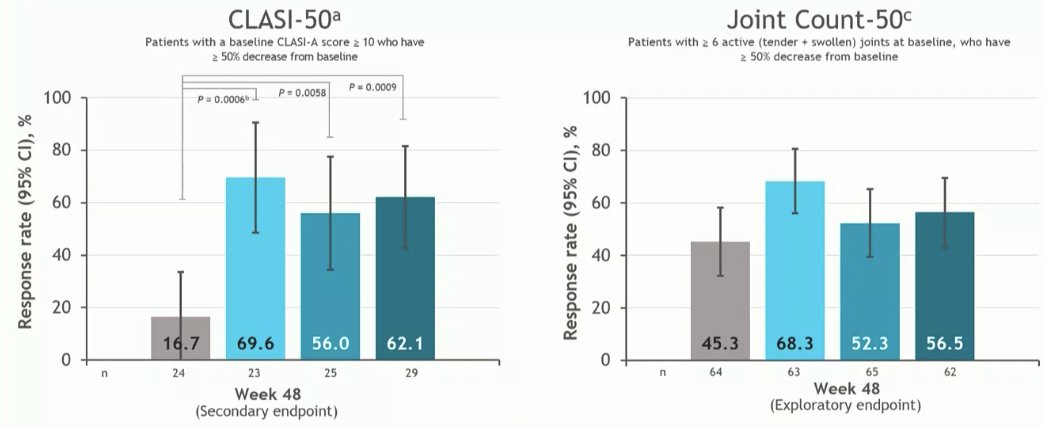
PAISLEY study Phase 2 RCT
Deucravacitinib in SLE meets primary endpoint wk 32
Results for dose 3mg BID:
*SRI(4) response 58.2% vs.
PBO 34%
*LLDAS 36%
*CLASI 70%
*⬇️ SJC
No new safety signal
Now waiting for Phase 3 👀
@RheumNow LB0004 #EULAR2022 #Lupus https://t.co/2KYSvfq4h7

1 year 10 months ago
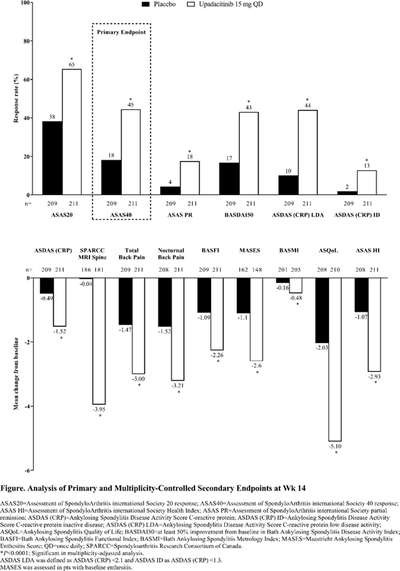
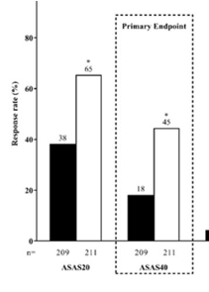
New in AS. Upadacitinib 15mg od was significantly more effective than placebo over 14 weeks in AS bDMARD-IR (ASAS40 45% vs 18% and the onset of effect seen by week 4), no new safety risks identified in the SELECT-AXIS 2 study by Van de Heijde et al #EULAR2022 @RheumNow POS0306 https://t.co/LCQBf38GWe

1 year 10 months ago
SELECT-AXIS 1 trial of upadacitinib in active #AS with an inadequate resp to prior biologic - pivotal phase 2/3 RCT of 420 AS pts (Dz dur 7.7 yrs; 83% B27+) showed better ASAS40 at wk 14 w/ UPA vs PBO (45% vs 18%; P<0.0001) #EULAR2022 POS0306 https://t.co/6ZBEvjRjqc https://t.co/GL0vlB34mE

1 year 10 months ago
Please see my short video on Promising Safety Outlook for Rituximab and Baricitinib in Vaccinated Patients #EULAR2022 @RheumNow https://t.co/bqzdWIzoOg

1 year 10 months ago
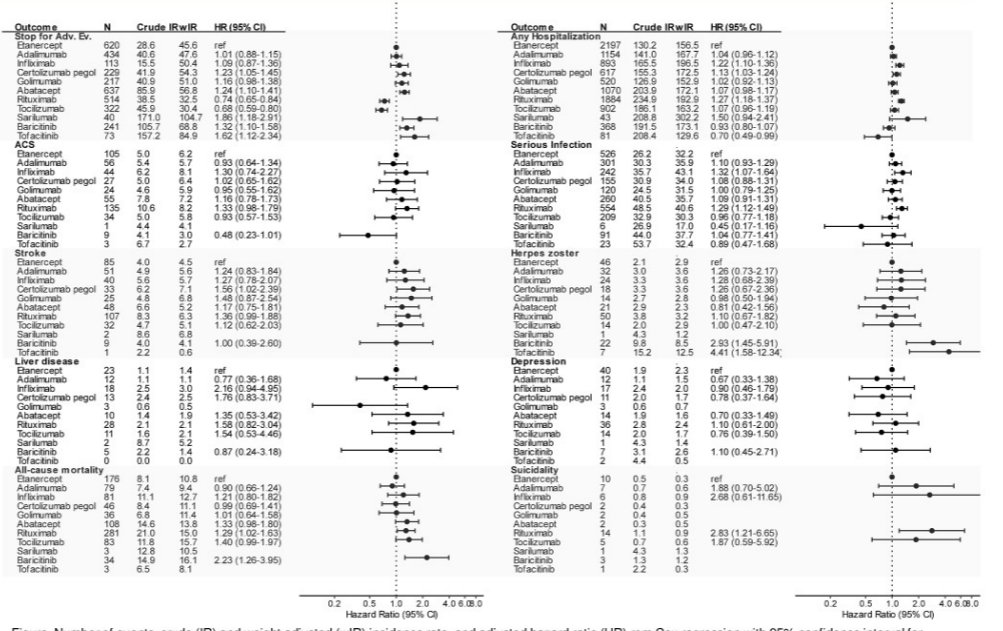
Frisell et al. Safety b/tsDMARDs from 10 years ARTIS. There is a mass of interesting data here, look at that figure! Higher rates of discontinuation due to AEs for tofa, bari, sari, rituximab. May be explained by chanelling and residual confounding @RheumNow #EULAR2022 POS0637 https://t.co/KrspU6c9Cs

1 year 10 months ago
What drives residual pain improvement in #JAKi treated pts? Dunno but #Baricitinib and #Sarilimab showed better pain decrease vs placebo and #Adalimumab. #OP0052 showed both #Tofacitinib & Adalimumab reduced pain more than placebo in PsA & RA if in remission @RheumNow @eular_org















 Poster Hall
Poster Hall