Novel Rx

Dr. John Cush RheumNow
2 years 3 months ago
FDA has approved another ustekinumab biosimilar (Wezlana) as being interchangeable for use in adults with plaque psoriasis, psoriatic arthritis, Crohn’s disease and ulcerative colitis. https://t.co/zbQKjmKgZh https://t.co/UuTDRgCBIV


Dr. John Cush RheumNow
2 years 3 months ago
Ph 3 360 pt RCT of sarilumab in #GCA was terminated early (COVID). 83 pts Rx'd, only 35%(29) completed. Sustained remission @wk 52:
- SAR 200: 6/13
- SAR 150: 3/7
- PBO: 0/6
All had 26wk GC taper
Sm sample size & COVID ruined RCT interpretation https://t.co/axOvJGpUti #PMR https://t.co/Xpabxn1IjZ

Dr. John Cush RheumNow
2 years 3 months ago
Subcutaneous Biosimilars?
Dr. Cush reviews RheumNow's top entries the #PMR Campaign, news, journal reports and regulatory actions.
https://t.co/BkKFbv0I64 https://t.co/EABeZxCKYZ

Despite the official recognition of PMR as a distinct disease more than 60 years ago, patients with PMR are still largely treated with steroids (glucocorticoids, mostly prednisone). The persistent broad use of glucocorticoids in PMR is related to their quick initial efficacy in the majority of patients with PMR, their low price and the lack of alternative treatments and paucity of glucocorticoid-sparing treatments.

Dr. John Cush RheumNow
2 years 3 months ago
Subcutaneous Remsima, an infliximab biosimilar, has been FDA. Celltrion has rebranded this as Zymfentra, for maintenance therapy in adults with moderately to severely active Ulcerative colitis and Crohn's disease following (after giving IV infliximab) https://t.co/3hb5Z7L9uw https://t.co/4gvSurl1nM
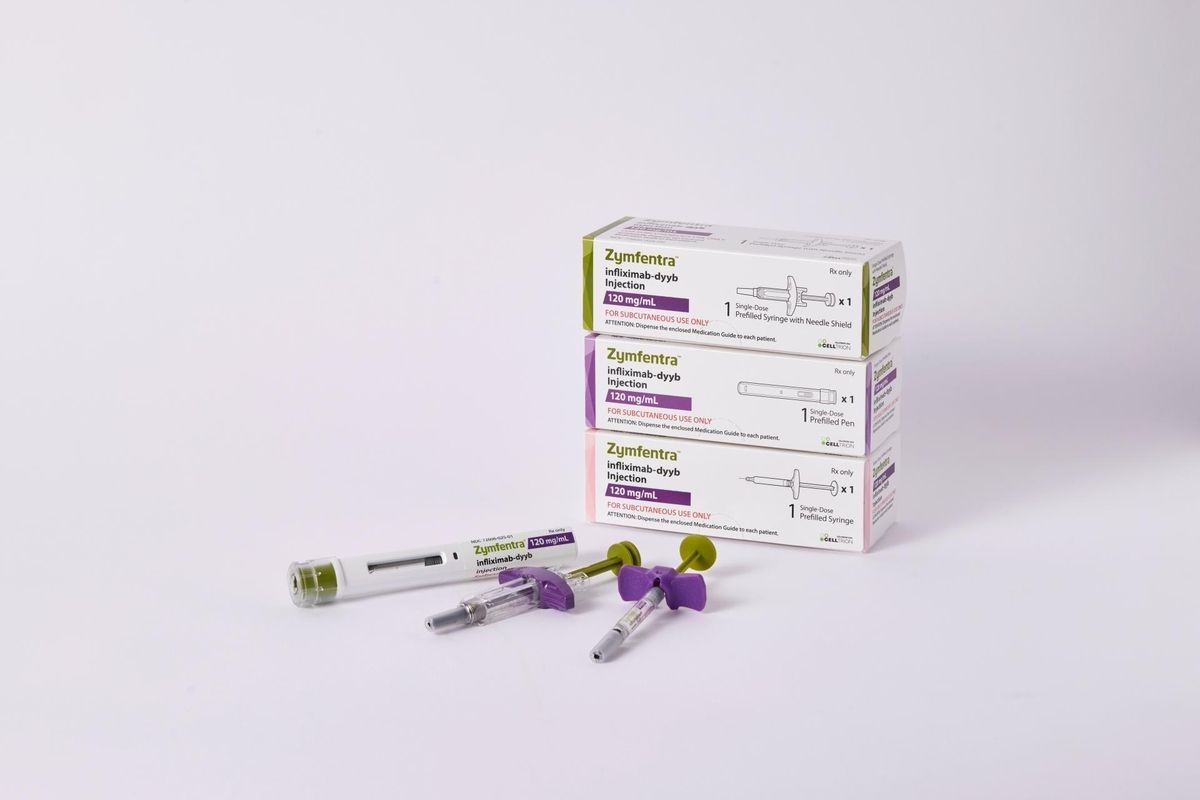

Dr. John Cush RheumNow
2 years 3 months ago
RheumNow Live registration is NOW OPEN! Early bird pricing through 11/30/2023. Register today!
https://t.co/7OsdPYWukv https://t.co/QoCxR04bJD

Another infliximab biosimilar has been FDA approved, but this new version of Inflectra can be given subcutaneously for patients with inflammatory bowel disease.
Dr. Jack Cush reviews RheumNow's top entries the PMR Campaign, news, journal reports and regulatory actions.
#PMR Polymyalgia rheumatica is very common, yet there is little research on the Dx & Management of PMR by #PCPs! Mayo study prevalence 701 per 100,000 (women=870 and men =508 per 100,000 population). https://t.co/7JZFeRPRnn

Dr. John Cush RheumNow
2 years 3 months ago
FDA Approves Bimekizumab for Plaque Psoriasis
UCB announced today that the FDA has approved bimekizumab-bkzx (Bimzelx)) for the treatment of moderate to severe plaque psoriasis in adults who needing systemic therapy or phototherapy.
https://t.co/BqeSAAxtQb https://t.co/LFjzkmAC5w


Dr. John Cush RheumNow
2 years 3 months ago
Bimekizumab (Bimelex), a dual IL-17A/F inhibitor, is FDA approved for u Treatment of Adults with Moderate to Severe Plaque Psoriasis https://t.co/Eo4LMaTHxn https://t.co/abzlThasEN

Dr. Jack Cush discusses the news, journal articles and regulatory actions. This week we discuss JAKne, DLE and SLE and more.












 Poster Hall
Poster Hall