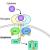
JAK/TYK2
There are several new positive RCTs for JAK inhibitors. Here is a summary of trials and new indications for JAKi in GCA, pediatrics, GI, dermatology and more.
The final day of EULAR 2024 was rich in posters, Late-breaking oral presentations and EULAR updates and recommendations. Below is a synopsis of the half-day's action.
A clinical trial in early axial spondyloarthritis (axSpA) demonstrated sustained inactive disease status in over 60% of patients. In axSpA patients with less than 1 year of disease symptoms, male sex, abstinence from smoking and lower BASDAI score at baseline were associated with higher chances of remission. Despite stable inactive disease state successfully induced by medication, drug-free remission in axSpA remains challenging.
There are a number of diseases where new biologic and targeted synthetic therapeutic options are coming online, and the temptation will be to consider them all equally. GCA is one of those diseases.
The development of JAK inhibitors represents an advance for the treatment of inflammatory rheumatic diseases. However, the enthusiasm in using this target has waned since the publication of the ORAL surveillance clinical trial in 2022. Subsequently, both the FDA and EMA issued a warning on using JAKi in patients with certain risk factors, e.g., ≥65 years, at increased risk of MACE, current smoker or past smoker and at increased risk of cancer,
Day 2 at EULAR 2024 was a big poster day for many with several good sessions and oral presentations on Preventing RA, new vasculitis therapies and a session devoted to the 50th anniversary of the Moll & Wright Criteria.
Dr. Jack Cush reviews FDA approval, news and journal articles -- and it’s the week before EULAR 2024 in Vienna!!














 Poster Hall
Poster Hall