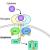
Anti-Rheumatic Rx
An exhaustive, full read guideline from the British Association of Dermatologists (BAD) and the British Society for Rheumatology (BSR) on the management and treatment of Behçet’s disease (BD) in 2024 has been published in Rheumatology.
Many conventional synthetic, biological, biosimilar, and targeted synthetic disease-modifying anti-rheumatic drugs are currently commercially available for the effective treatment of rheumatoid arthritis. However, the "Holy Grail" remains the prevention of its development. During a clinical symposium at EULAR 2024 in Vienna, updates were presented on four prospective intervention trials conducted in patients experiencing joint pain without
Fertility in the context of RMDs is a daily concern for a lot of rheumatologists and patients.
EULAR this year proposed new fertility data, especially in Rheumatoid Arthritis, along with an update of the EULAR points to consider for use of antirheumatic drugs in reproduction, pregnancy and lactation.
Autologous hematopoietic stem cell transplantation (aHSCT) for SSc has been proven the most effective treatment strategy with regard to overall and event free survival in selected patients.2 But a key limitation is its toxicity, and new treatment options are needed. Two abstracts presented at the 2024 EULAR congress in Vienna focused on novel approaches.
The final day of EULAR 2024 was rich in posters, Late-breaking oral presentations and EULAR updates and recommendations. Below is a synopsis of the half-day's action.
A clinical trial in early axial spondyloarthritis (axSpA) demonstrated sustained inactive disease status in over 60% of patients. In axSpA patients with less than 1 year of disease symptoms, male sex, abstinence from smoking and lower BASDAI score at baseline were associated with higher chances of remission. Despite stable inactive disease state successfully induced by medication, drug-free remission in axSpA remains challenging.
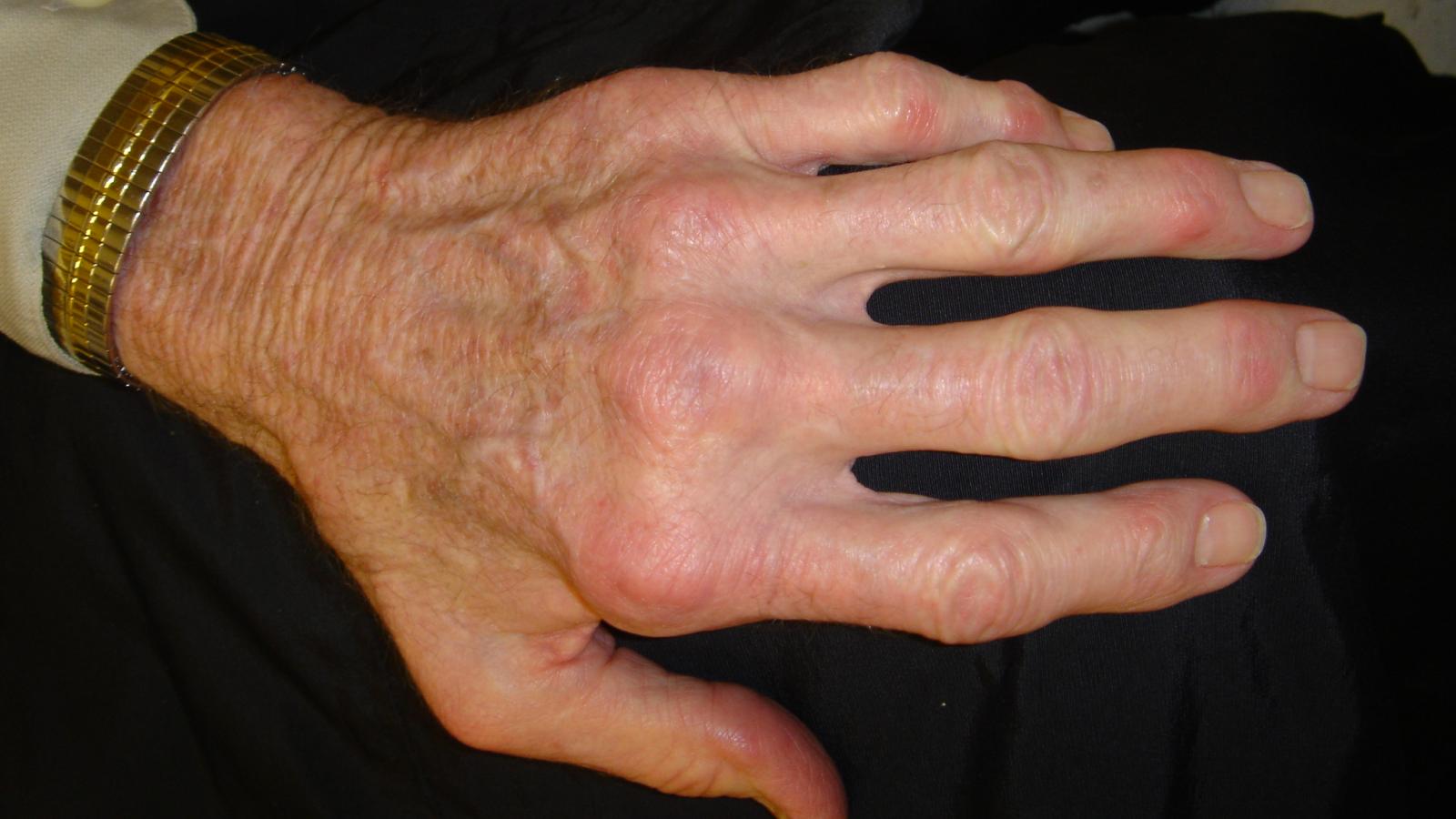
It is almost four years since EULAR published its definition of difficult-to-treat (D2T) rheumatoid arthritis (RA) (1). This marked a formal recognition of the group of patients who, despite the advances in pharmacological therapy for RA, remain symptomatic.
EULAR treats its attendees to a wonderful lunch each day – Schnitzel, gnocchi, goulash at lunch after muffins and coffee in the morning. It's no wonder the meeting has been a lively one, well attended with a wealth of stimulating ideas, research and discussion. Here are a few of my favorite abstracts and presentations from today, day 3 in Vienna.
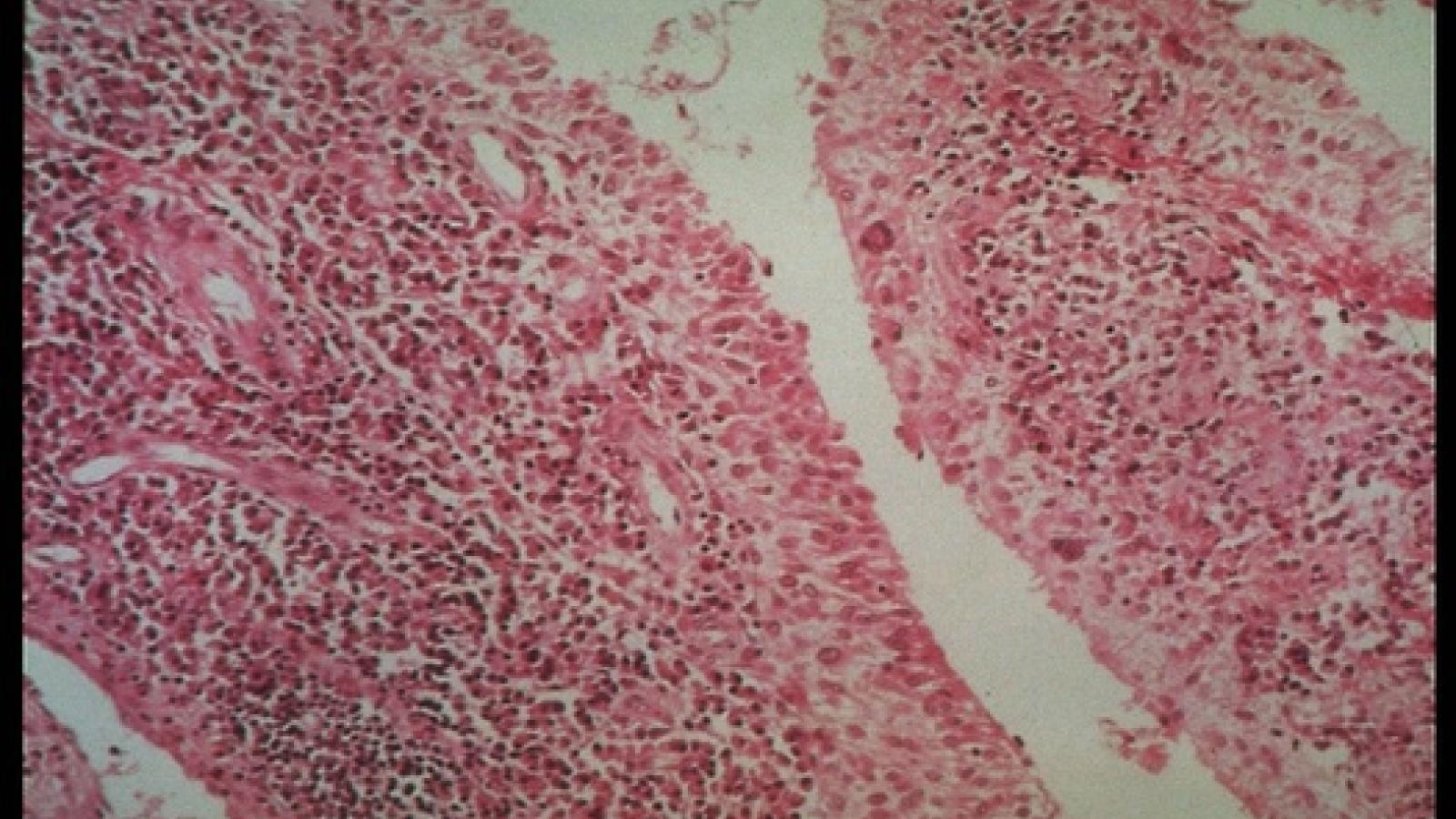
Multi-modal analysis of rheumatoid arthritis (RA) synovitis pathotypes enables more accurate choices of first line cDMARDs treatment, suggesting a predictive value to synovial biopsy.
Day 2 at EULAR 2024 was a big poster day for many with several good sessions and oral presentations on Preventing RA, new vasculitis therapies and a session devoted to the 50th anniversary of the Moll & Wright Criteria.
Analysis of French claims data, found over half of women initiating oral bisphosphonates (BP) between 2015 and 2020 discontinued treatment for at least one year, which was associated with a 12% increase in fracture risk. While not recommended, 42.5% of women initiating Dmab discontinued treatment for at least one year, which almost doubled their fracture risk. No increased risk was observed for long term discontinuation of IV BP.
Dual seropositivity (rheumatoid factor and/or anti-citrullinated protein antibodies) as well as shared epitope are known to be associated with poor prognosis and structural damage in RA.
Wednesday was Day One at EULAR 2024 in Vienna. While the day was a slow start, the poster halls and auditoriums quickly filled with thousands of rheumatologists, eager to reunite at this international educational forum. Below are a few of my favorites from Day 1.
Just about every Medicare Part D prescription drug plan on offer this year covers the original version of adalimumab (Humira), whereas only about half will pay for any of the several biosimilar versions currently approved, researchers found.

















 Poster Hall
Poster Hall