JAK/TYK2

Aurelie Najm
2 years 1 month ago
Insights into pathogenesis venous & arterial thrombosis in RA + JAKi
Significant dysregulation of clotting pathways in myeloid & increased clot formation in context of microbial challenge (TLR4)
Observed across all JAKI, but selective JAK3i
@RheumNow #ACR23 ABST1676 https://t.co/nboy0znfAW


Dr. Antoni Chan
2 years 1 month ago
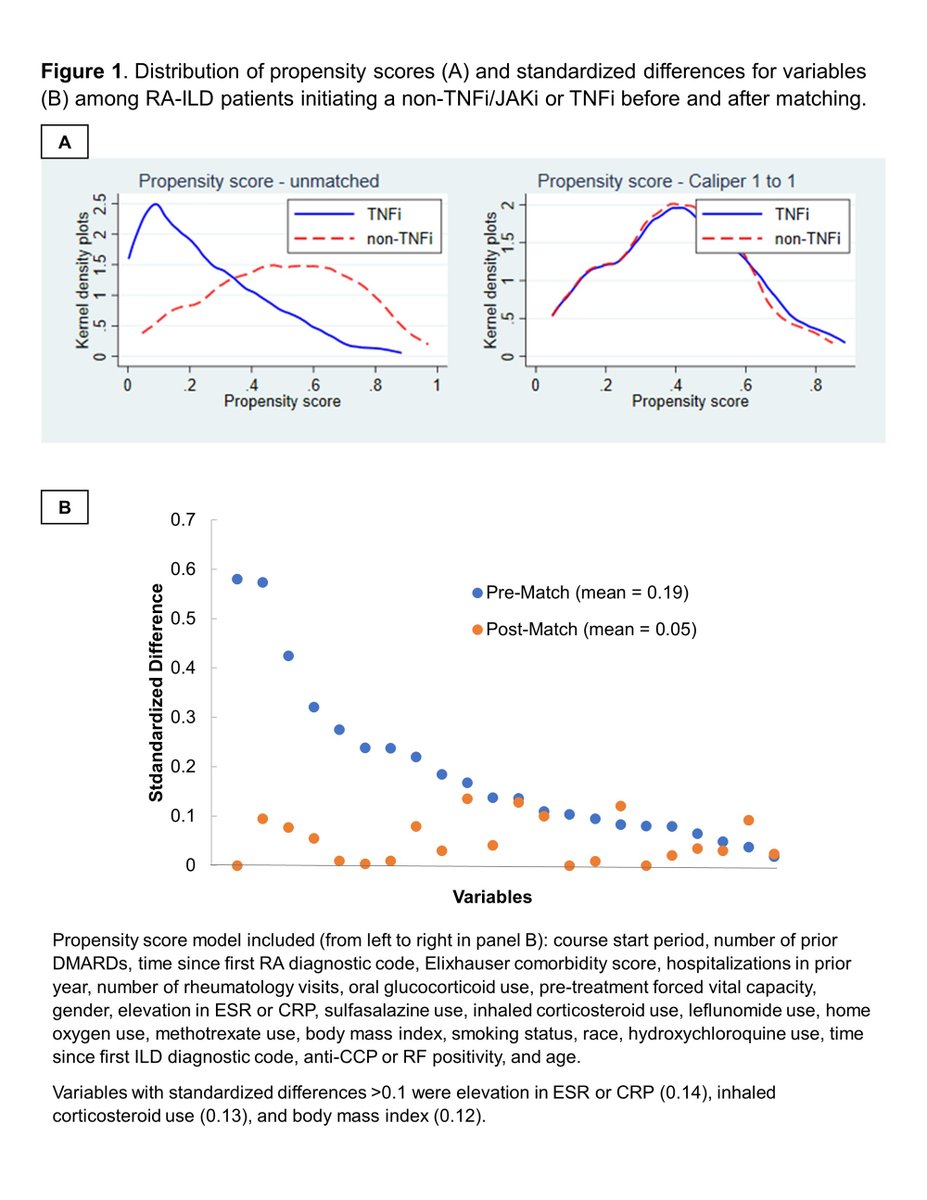
In PS-matched study, no significant difference in respiratory hospitalisation or death between RA-ILD patients on non-TNFi/JAKi vs. TNFi. This finding does not support systematic avoidance of TNFi in RA-ILD, England B Abst#1582 #ACR23 #ACRBest @RheumNow https://t.co/1zVjMWNZZS https://t.co/d8TRIvGaIe

TheDaoIndex KDAO2011
2 years 1 month ago
Debaters and moderators acknowledge IL6i may not be the only steroid sparing agents that would work for #GCA. Abatacept, secukinumab, JAKi are being evaluated #greatdebate @rheumnow #ACR23

Mike Putman EBRheum
2 years 1 month ago
This is pretty nerdy, but I would love a documentary about filgotinib
JAK class wins/fails feel highly idiosyncratic to me
The difference between UPA & FIL from science perspective seems negligible; financially, it's many billions of $$$
@rheumnow #ACR23 Abstr 1325 https://t.co/efGwSSrxz7
The RheumNow faculty reporters have been scouring the meeting for what they believe to be the best presentations from the first day at ACR 2023 in San Diego. From hundreds of online presentations, the poster floor and the plenary podium, here are some of the best abstracts from Sunday Nov. 12th. You can spot these on Twitter by looking for the (#ACRbest) hashtag.

Mike Putman EBRheum
2 years 1 month ago
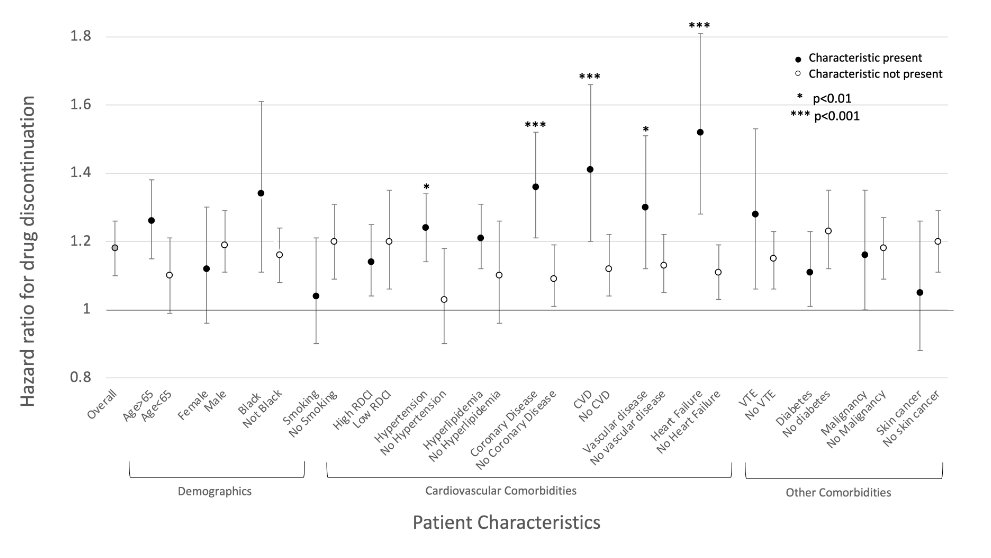
Super interesting study re: impact of ORAL SURVEILLANCE on prescribing
Post safety report, JAK pts shifted younger w/lower comorbidities (esp CVD)
I know many have mixed feelings, but I believe in the risk & support this type of shift
@RheumNow Abstr0435 #ACR23 https://t.co/EBg9VOIbj1

Meral K. El Ramahi, MD MeralElRamahiMD
2 years 1 month ago
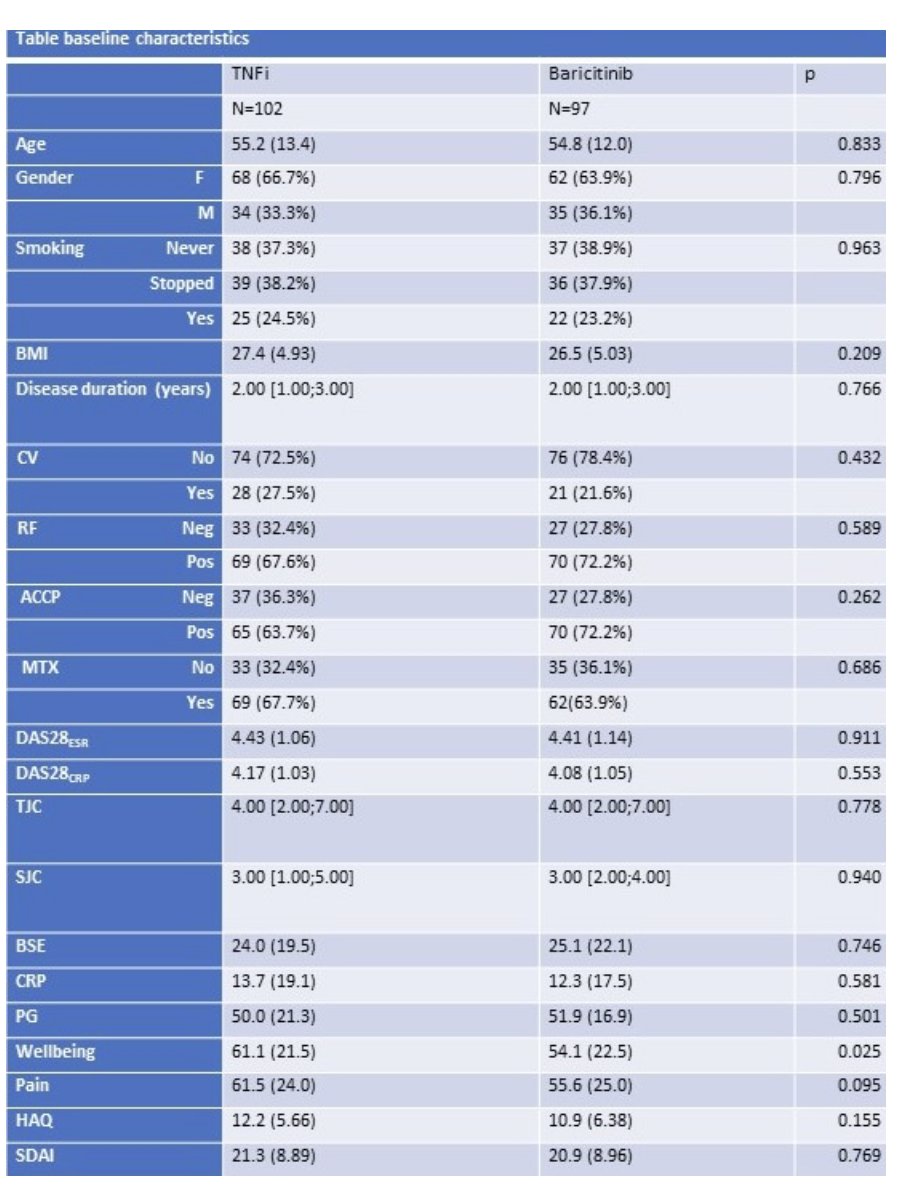
ABS0450
⭐️Baricitinib non-inferior as well as superior to TNFi in terms of ACR50 response @ 12w in real world csDMARD refractory RA patients
➡️open-label, T2T w/ Baricitinib (n=102) vs TNFi (n=97)
➡️DAS28-CRP remission (<0.6) in 74% of Bari vs 47% of TNFi
#ACR23 @RheumNow https://t.co/yydS20hNzM

Mike Putman EBRheum
2 years 1 month ago
No more underpowered long-term safety studies
We need a "SELECT-SURVEILLANCE" study to replicate ORAL-SURVEILLANCE & tell us if UPA has the same MACE/cancer risk as TOFA
Minus that, I plan to go 100% TOFA when it becomes generic in 2026
@RheumNow #ACR23 Abstr1326 https://t.co/LyQ1Q8qReO


Dr. Rachel Tate
2 years 1 month ago
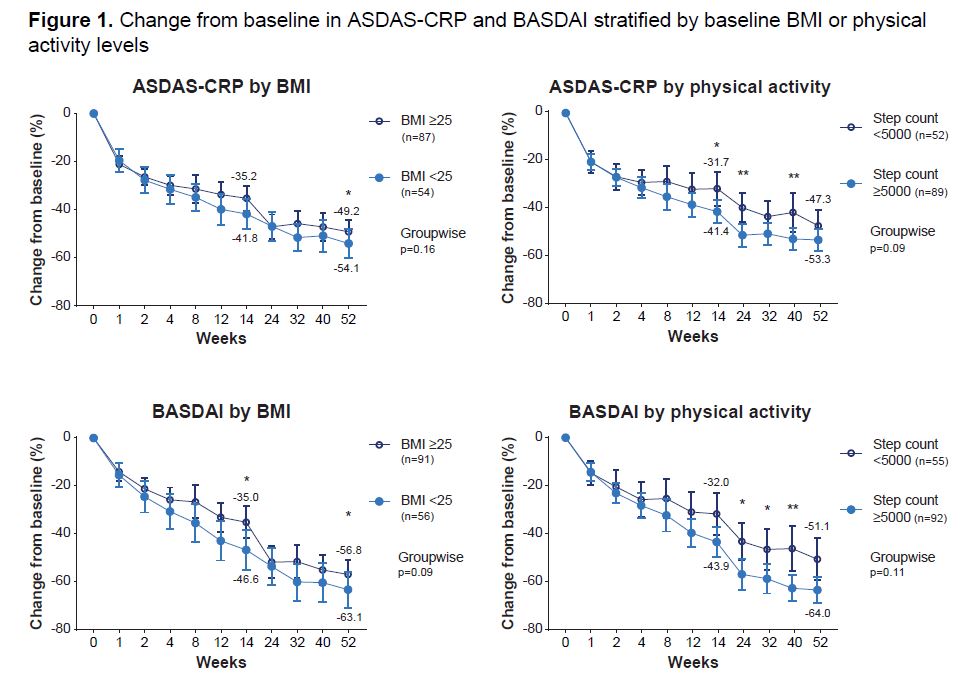
AS pts on UPA for 52 wks who were active or healthy weight/underweight at BL generally experienced greater decreases from BL in ASDAS-CRP and BASDAI vs pts who were inactive or overweight/obese. #ACR23 Abs #0540 https://t.co/OfgbmYUoAu @rheumno https://t.co/liptgbK2hJ

Caoilfhionn Connolly
2 years 1 month ago
🔥 Absolute masterclass @philseo in the Clinical Year in Review #ACR23
➡️ Certain subpopulations have lower risk of AE with Tofacitinib
➡️ Knee OA 📈 75% by 2050
➡️ Exciting novel approaches in Rx of IA in development
➡️Better clinical trial endpoints needed for SLE https://t.co/5cjbZLPsCM


Richard Conway
2 years 1 month ago
Phase 2 RCT of TLL-018 (JAK1/TYK2i) vs Tofa in RA. 101 patients. ACR50 72% vs 42%. 83% of tofa-IR achieved ACR50 on TLL-018. Can't wait for the phase 3 data! Abstr#0840 #ACR23 @RheumNow #ACRbest https://t.co/wjGx4Edxyb https://t.co/n6CL8G01Wo


David Liew drdavidliew
2 years 1 month ago
TLL-018, the dual JAK1/Tyk2 inhibitor which caused all the chatter at #EULAR2023, now with more full data showing it absolutely smash tofacitinib in RA, with similar safety.
“This is a spectacular compound - unbelievably spectacular” - Roy Fleischmann
#ACR23 ABST0840 @RheumNow https://t.co/nIKQb4yYrJ














 Poster Hall
Poster Hall