IL-17
The British Society of Rheumatology has published their updated 2022 recommendations for the use of biologics and targeted synthetic treatments in patients with psoriatic arthritis. These guidelines follow initial treatment with a single conventional systemic disease-modifying anti-rheumatic drug, typically methotrexate typically. They noted that up to 50% of people with PsA require biologic or targeted synthetic (b/ts)DMARD therapy.
Bags are packed, ready to go, but wait there’s more abstracts to show.
The big news today were the “late breaking” abstracts. This is usually a favorite session of many as this is where the newest of study data often is showcased.
Here are my favorite late-breakers from Day 4.
For autoimmune patients with a history of malignancy, the initiation of biologic or targeted synthetic disease modifying agents (bDMARD/tsDMARDs) may provoke concern. While data for biologic medications and malignancy risk has been largely reassuring, clinical trials have often excluded patients with history of cancer.
At EULAR 2022, I have been looking at topics and presentations in psoriatic arthritis (PsA). Here are my top picks from this year's meeting.

Robert B Chao, MD doctorRBC
3 years 8 months ago
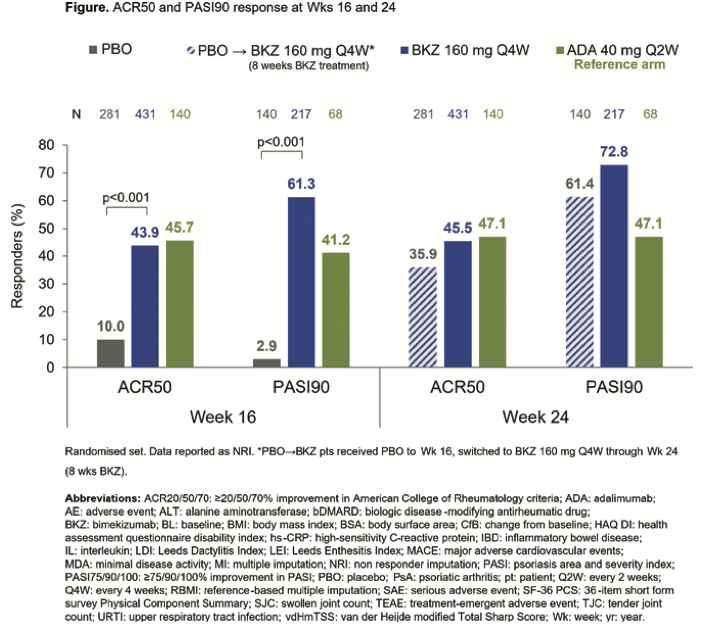
Bimekizumab: IL-17A and F inhibitor for tx of PsA - BE OPTIMAL trial reached primary endpoint: ACR50 44% vs. placebo
Efficacy as early as 2 weeks
No MACE, uveitis, IBD, deaths
@RheumNow #EULAR2022 ABST#LB0001 https://t.co/sHRRwLlChi

Robert B Chao, MD doctorRBC
3 years 8 months ago
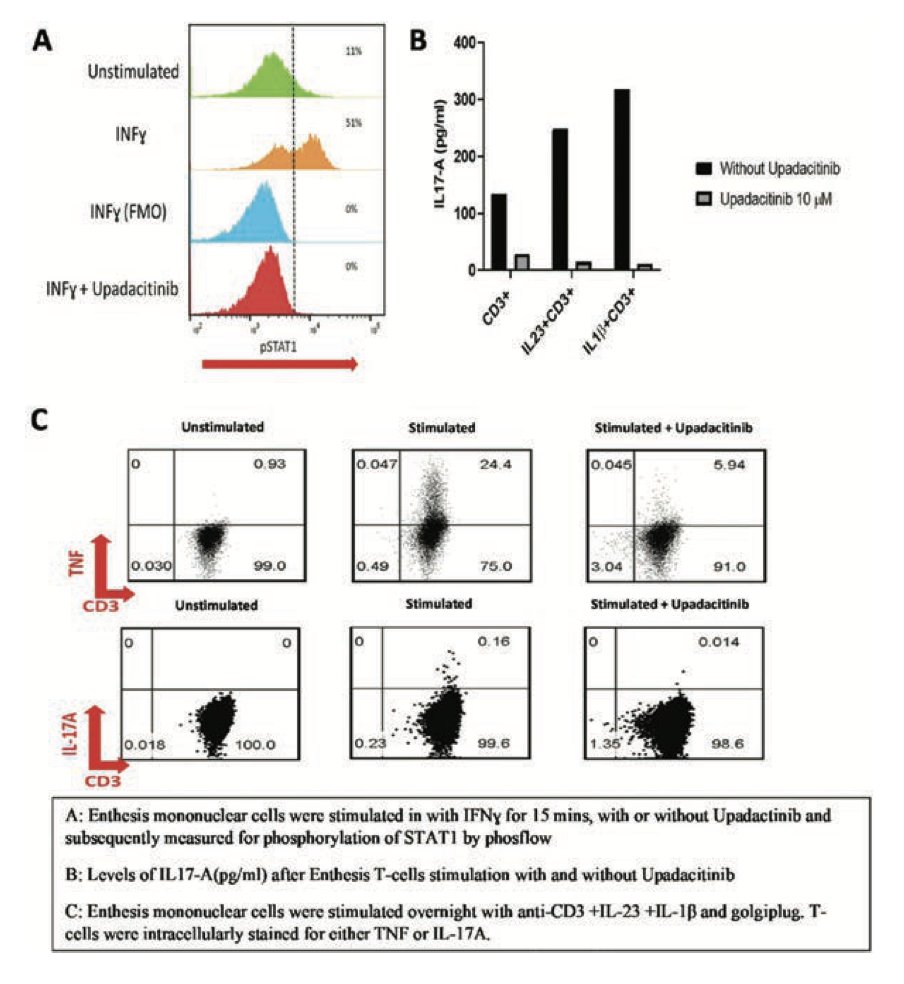
In vitro study shows Upadacitinib inhibits enthesis T cell derived TNFa and IL-17A, disrupting the prominent IL-23/IL-17/TNFa axis driving SpA.
@RheumNow #EULAR2022 ABST#POS0331 https://t.co/pNL3Zlgmt7

TheDaoIndex KDAO2011
3 years 8 months ago
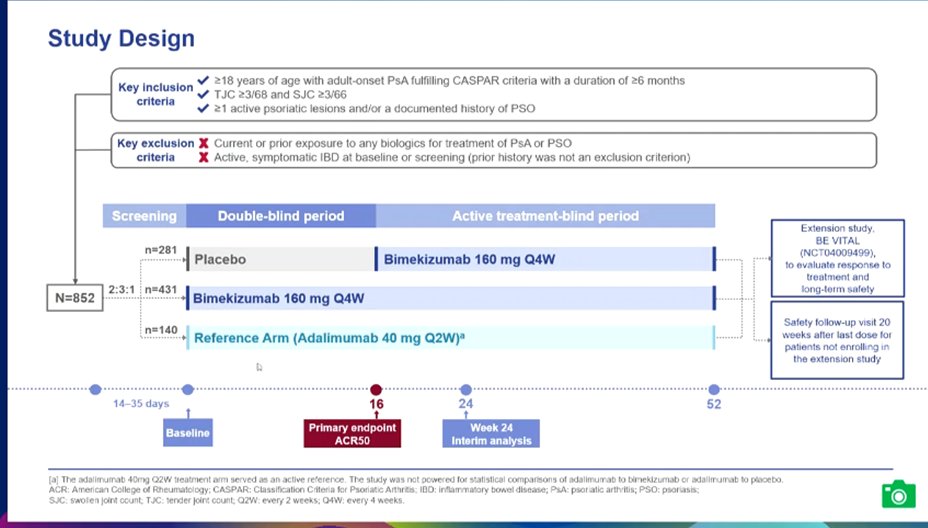
Phase 3 compared Bimekizumab to PCB and Humira; it met all endpoints for PsA; the drug inhibits both IL17F & IL-17A. Safety signal: increase fungal (candidal) infx #EULAR2022 LB0001 @rheumnow https://t.co/MJJwj44YgU

Richard Conway RichardPAConway
3 years 8 months ago
McInnes @IainBMcInnes1 et al. Bimekizumab (IL17A/Fi) in PsA. BE OPTIMAL 852 patient RCT vs PBO vs ADA. Looks same for joints, better for skin than ADA (not stat sig) @RheumNow #EULAR2022 LB0001 https://t.co/oKEHJE3tGn https://t.co/H7nPnbEWUB

Dr. Antoni Chan synovialjoints
3 years 8 months ago
Bimekizumab (BKZ) vs PBO at 16 week BE COMPLETE for PsA with inadequate response to TNFi ACR50 44% vs 7%,PASI90 69% vs 7%, ACR20 67% vs 16%, ACR70 27% vs 0.8%, PASI 100 60% vs 5%, MDA 44% vs 6%, no new safety signals #EULAR2022 @RheumNow OP0255 Merola et al https://t.co/riU2sIXGs9
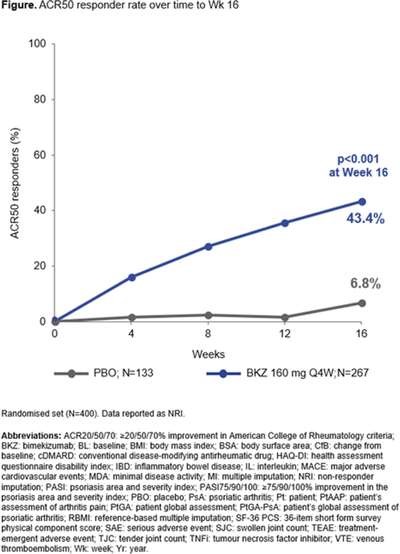

Md Yuzaiful Md Yusof Yuz6Yusof
3 years 8 months ago
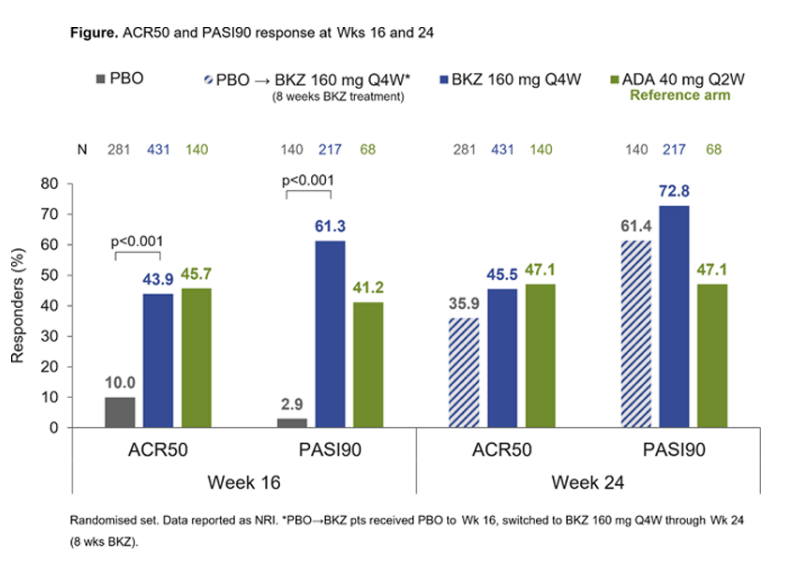
#LB0005 #EULAR2022 Phase 3 RCT of Bimekizumab, dual IL17A and 17F inhibitor in bDMARDs-naive PsA showed significant improvement in ACR50 and PASI90 scores vs Placebo. No major safety signals. Skin response is excellent! @RheumNow https://t.co/yhgB8LGXh6

Eric Dein ericdeinmd
3 years 8 months ago
#EULAR2022 LB0001
Bimekizumab (IL-17F and IL-17A) in bionaive PsA
BE OPTIMAL phase 3 trial, 24 weeks
⭐️Met Primary endpt - ACR50
⭐️Met other ACR and PASI endpts
⭐️Safety: higher fungal infections
@RheumNow https://t.co/x8u6B93FNl

Aurelie Najm AurelieRheumo
3 years 8 months ago
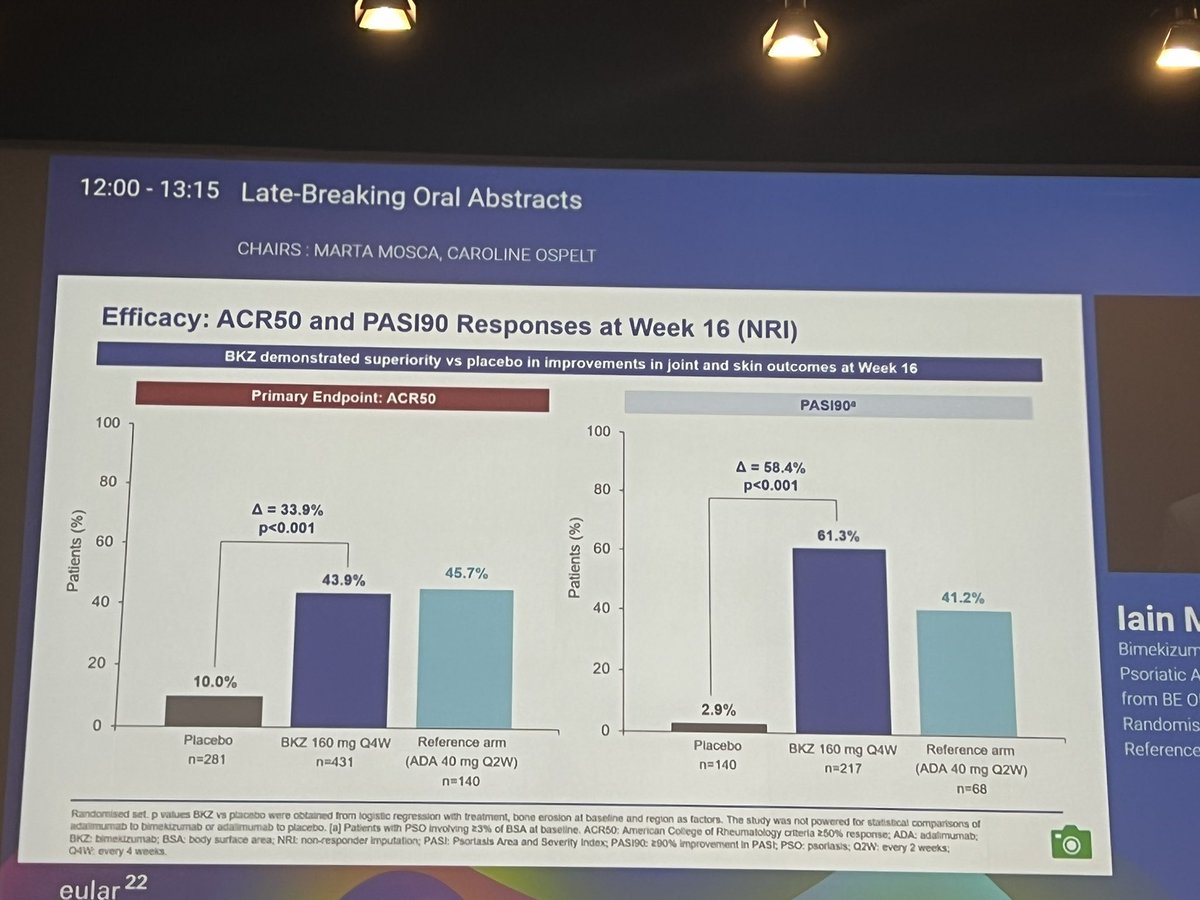
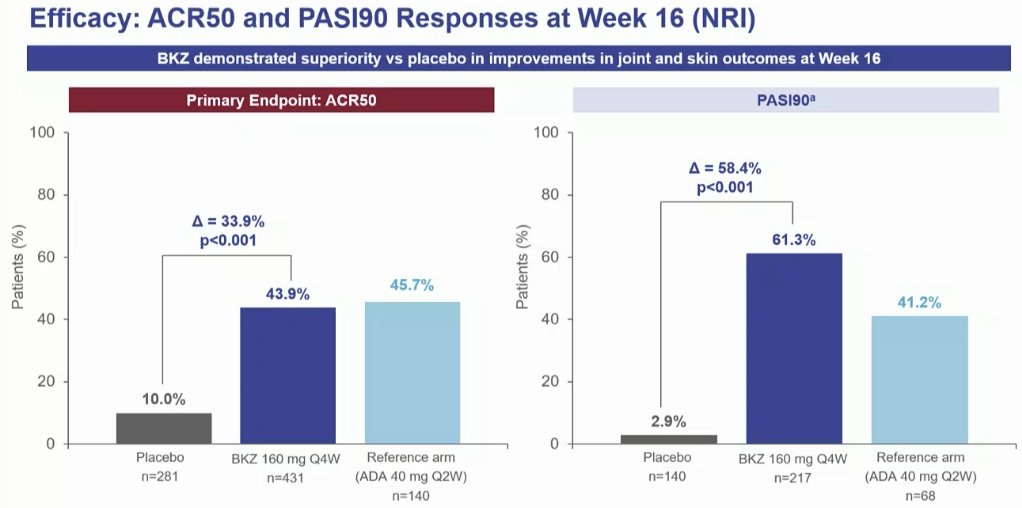
BE OPTIMAL Phase 3 RCT
Bimekizumab dual IL17A & F inhibitor in bionaive PsA
⭐️ACR50 wk 16 43.9% vs 10% PBO
⭐️PASI 90 wk 16 61% vs. 3% PBO
⭐️separation from wk 4
Safety: fungal injections BKZ > ADA
@RheumNow
LB0001 #EULAR2022 https://t.co/7ZwV1XDSNb
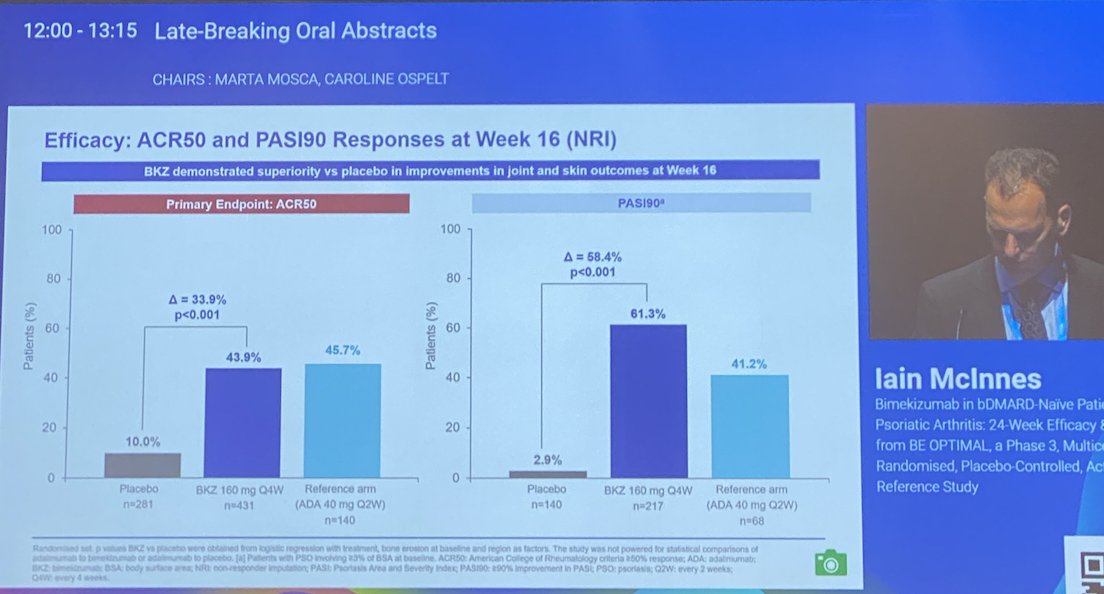

Aurelie Najm AurelieRheumo
3 years 8 months ago
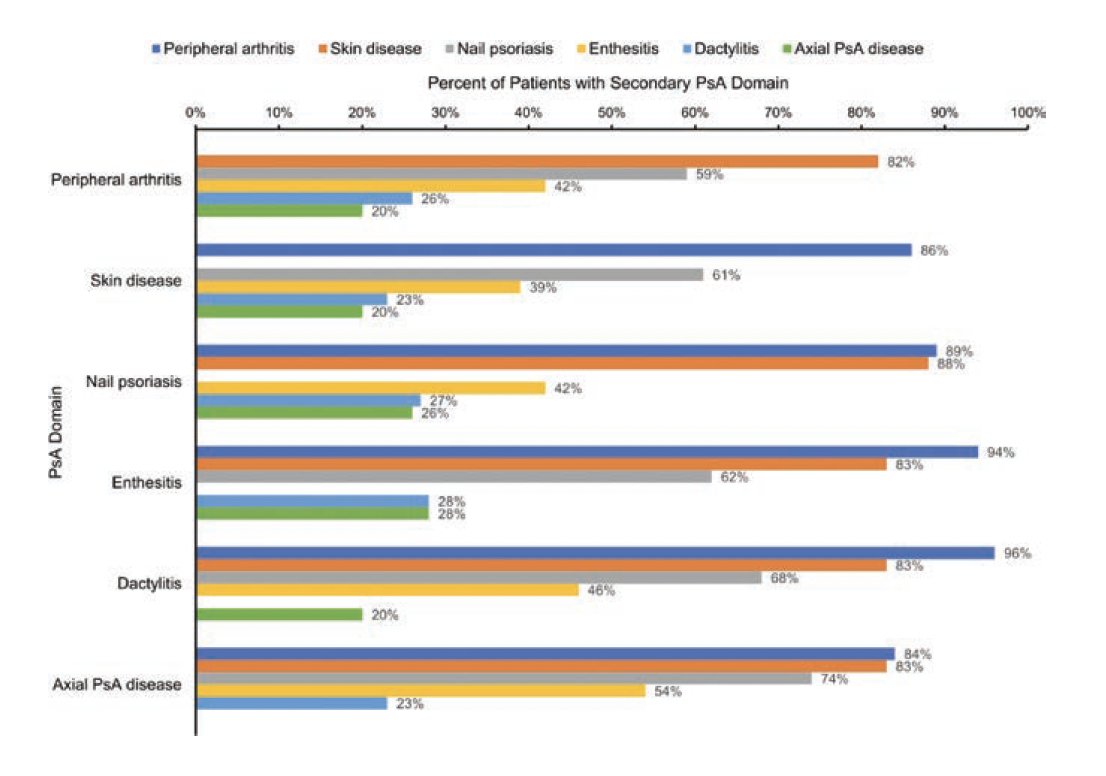
CorEvitas PsA Registry (1000+ pts)
Analysis of biologics prescription according to disease domains
No real surprise to see that IL-17i were prescribed more frequently in pts w/ PsO BSA>10% at BL
Overall first line TNFi 40% > IL-17i 14%
POS0309 @RheumNow #EULAR2022 https://t.co/dkLxl8d6ug

Richard Conway RichardPAConway
3 years 8 months ago
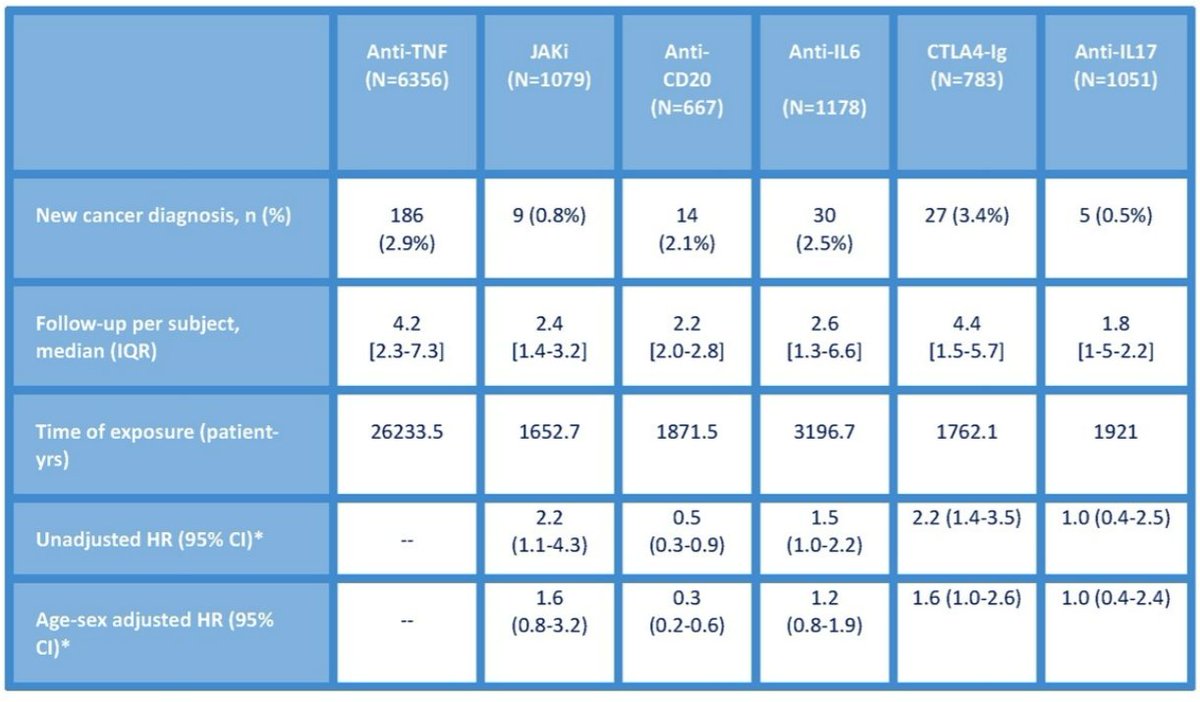
Castrejon et al. BIOBADASER registry study. Cancer risk with various bDMARDs. Overall I don't think there is a difference here. Lowest in IL17i and highest in abatacept but older and comorbidity confounders @RheumNow #EULAR2022 #POS1439 https://t.co/gfv78ouOTJ












 Poster Hall
Poster Hall