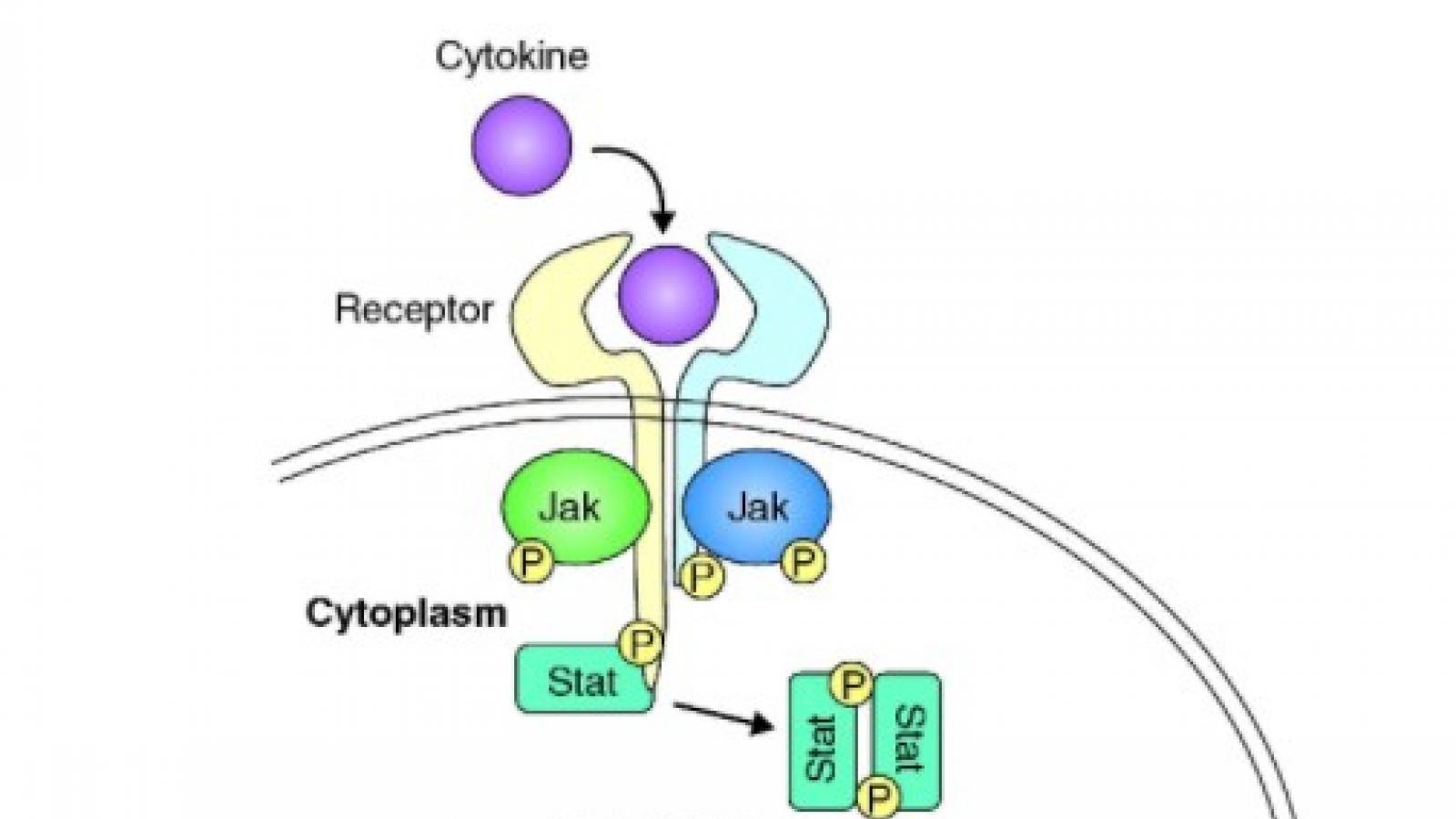
IL-17
The big news this week: the approval of deucravacitinib (a new class of drug?) for psoriasis; the 2022 ACR guidance on glucocorticoid-induced osteoporosis; a national poll of older adults over the age of 50 who claimed self-reported or doctor-diagnosed arthritis; and much more. Let's review these and other news, journal reports and announcements from this past week.
JUNIPERA study evaluated secukinumab (SEC) in children with enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) and was found to be safe and effective in patients with active ERA and JPsA who previous failed to respond to conventional therapy.
We've got a lot to discuss this week: psoriasis; fatigue; sleep; sural nerve biopsies; uveitis and SpA; diet and RA; tofacitinib and the ORAL surveillance study; what not to take with mycophenolate - and more. In what order should these items be discussed? This week the run down is based on popularity, measured by rheumatologist engagements on the website and social media.
Patient-reported fatigue is high in patients with psoriatic arthritis (PsA) and often goes under-recognized by physicians. Fatigue importantly impacts physical functioning, work productivity, and health related quality of life (HRQoL).
The ACR has posted a new ACR Clinical Practice Guideline Summary providing recommendations on the use of vaccinations for children and adults with rheumatic and musculoskeletal diseases (RMDs).
This guideline builds on past ACR vaccination guidance, last published in 2021.
Dr. Jack Cush reviews the new studies and drug approvals and new insights into febrile disorders from the past week on RheumNow.com
Ustekinumab (IL-12/23i) phase 2 study was successful in #SLE; but the phase 3 LOTUS study of 512 pts, UST was NOT superior to PBO (SRI-4 44% v 56%) at 1 year. Study was halted for lack of efficacy (but no safety concerns). https://bit.ly/3RvPzDm
With the recent publication of the third iteration of the GRAPPA Psoriatic Arthritis (PsA) treatment recommendations, it seems to be an auspicious time to reflect on some key considerations that arose during the development of the recommendations, as well as to look towards what the future may hold.
For 2022, let's look our top 10 list of advances, game-changers, worries and those better medical practices that evolved during 2021.
With their publication in June 2022 (1), the 3rd iteration of the Group for Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Treatment recommendations for Psoriatic Arthritis (PsA) may have set a record or sorts.
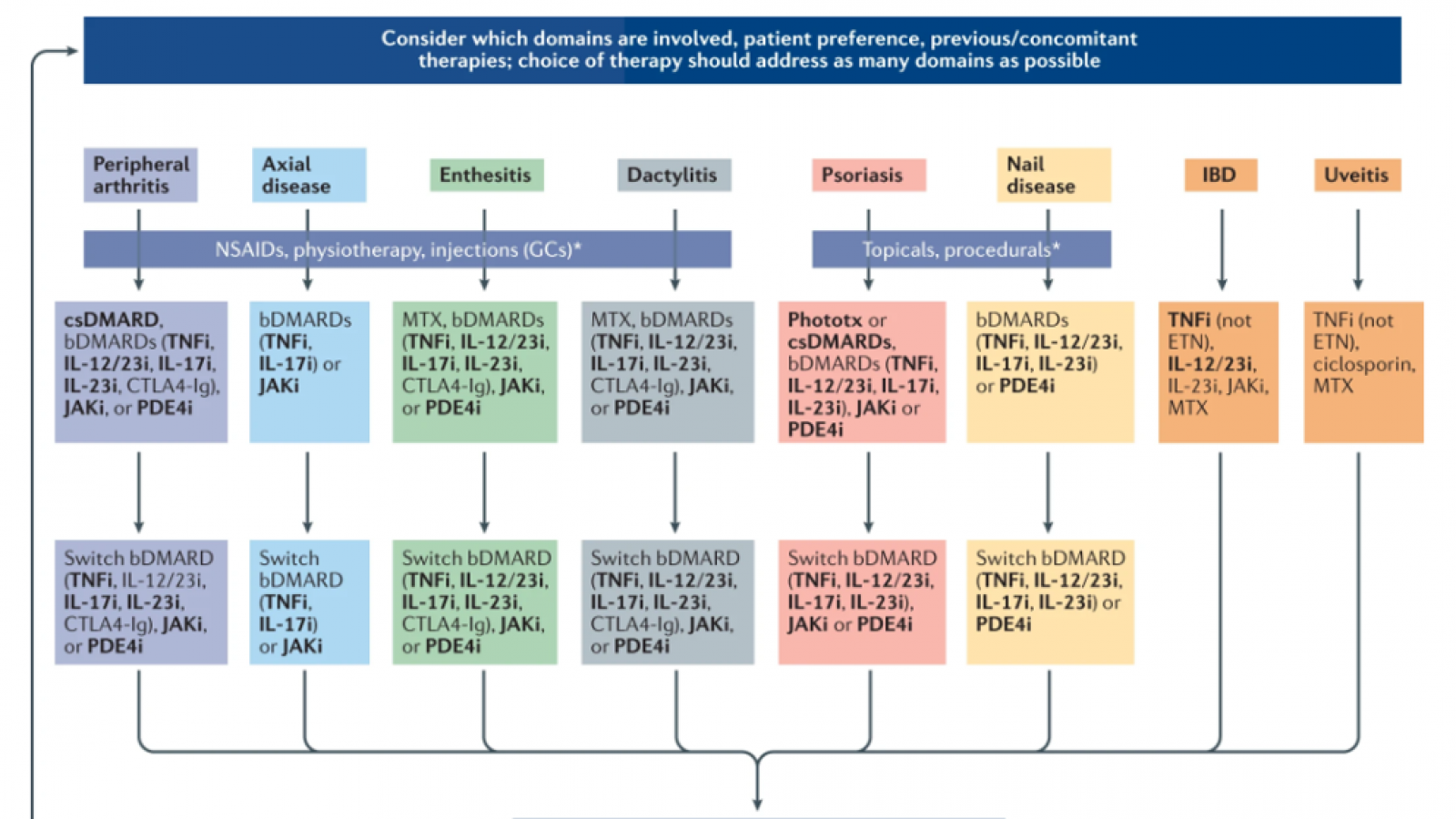
The GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) treatment recommendations have been updated and the 2021 evidence-based guidance is rich in treatment recommendations based on the key disease "domains" - peripheral arthritis, axial disease, enthesitis, dactylitis, skin and nail psoriasis; with new PsA related domains uveitis and inflammatory bowel disease.
Pooled data from two large ixekizumab (IXE) trials show that male patients had greater clinical responses than did female patients with psoriatic arthritis are largely unexplored. Reasons for this differential treatment responses to an interleukin-17A inhibitor (IXE) between male and female patients is unexplained.
A pooled analyses of data from eight, phase 2 and 3 randomized clinical trials shows bimekizumab, a dual IL-17A/F inhibitor, to be effective in plaque psoriasis and was well tolerated aside from an increased incidence of mild to moderate oral candidiasis.
Pyoderma gangrenosum (PG) is rare, but often associated with different forms of arthritis, in particular rheumatoid arthritis and inflammatory bowel diseases.
There are still questions surrounding COVID-19, and some common questions I receive from patients revolve around what to do with their current DMARDs or should they even start treatment during this pandemic. Two studies focused on this question.
The British Society of Rheumatology has published their updated 2022 recommendations for the use of biologics and targeted synthetic treatments in patients with psoriatic arthritis. These guidelines follow initial treatment with a single conventional systemic disease-modifying anti-rheumatic drug, typically methotrexate typically. They noted that up to 50% of people with PsA require biologic or targeted synthetic (b/ts)DMARD therapy.
















 Poster Hall
Poster Hall