
Dr. John Cush RheumNow
3 years 6 months ago
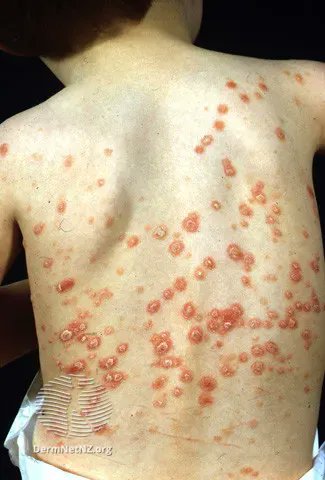
Risk of TNFi-related psoriasis in 5088 TNFi Rx children w/ IBD, JIA, or CNO. Compared to non-TNFi exposed, kids on TNFi had an increased PSO risk w/ ADA (IRR 2.70; 1.53-4.75), infliximab (2.34) or etanercept (2.21) & not incr w/ DMARD use https://t.co/Y1Vn9kjk5N https://t.co/5CCxa5kMcU

Dr. John Cush RheumNow
3 years 6 months ago
A Rheum & Derm Conversation on Treating PsA and Moderate-to-Severe PsO
Sponsored by Lilly USA
Listen to Philip Mease and April Armstrong discuss a multidisciplinary approach and importantconsiderations when treating patients with joint and
skin concerns.
https://t.co/lTfz2kUr51

Dr. John Cush RheumNow
3 years 6 months ago
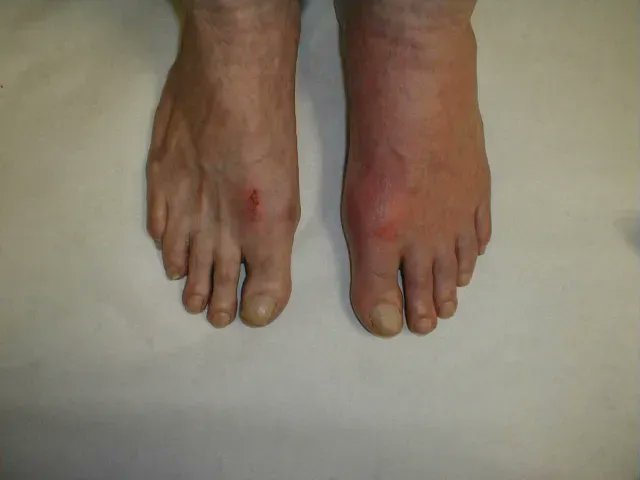
NICE Guidelines on Gout Diagnosis and Management
NICE (UK) has systematically reviewed current medical evidence and delivered a set of recommendations with consideration of cost effectiveness.
https://t.co/a8iyCe3szJ https://t.co/6Jy7dOT4yX

Dr. John Cush RheumNow
3 years 6 months ago
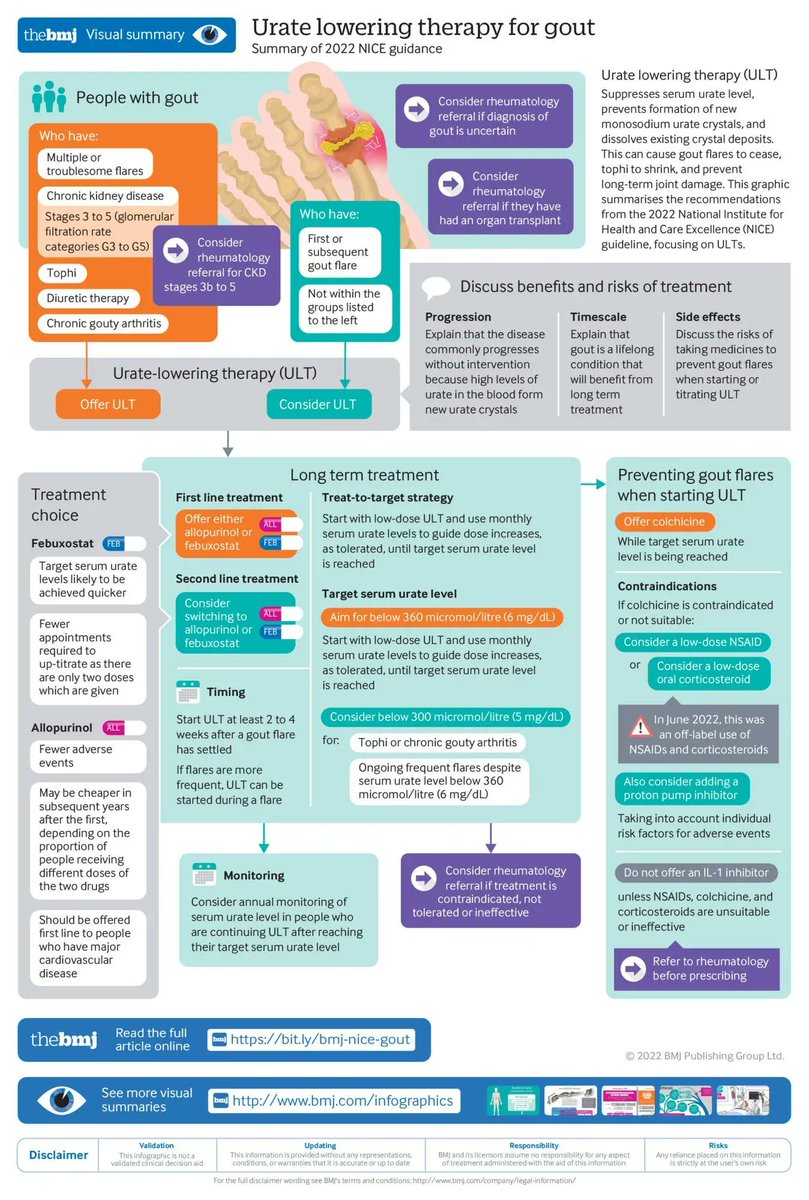
Gout: diagnosis and management—summary of NICE guidance https://t.co/tVHkSMusk6 https://t.co/A7U1DlI7tO

Dr. John Cush RheumNow
3 years 6 months ago
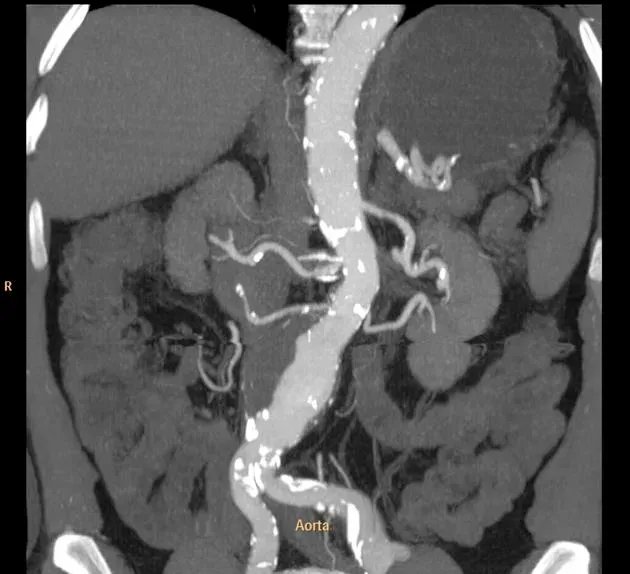
SURPRISED? Psoriasis pts have a known increased risk of CV disease. But did you know several papers & metanalyses show increased risk of abdominal aortic aneurysm in PSO (HR 1.30), worse w/ dz activity; lost w/ age, female, DM? https://t.co/9ihyubUgCI https://t.co/W1JiXV0yOX https://t.co/Nq6aVBjPuK

Dr. John Cush RheumNow
3 years 6 months ago
Review of IL-1 inhibitors in recurrent idiopathic pericarditis. While Rilonacept is FDA approved (320 mg x1 then 160 mg sc/wk), anakinra (2 mg/kg/d or 100 mg/d), canakinumab and colchicine are used https://t.co/j00yWpBbq3 https://t.co/7BK2mopssc
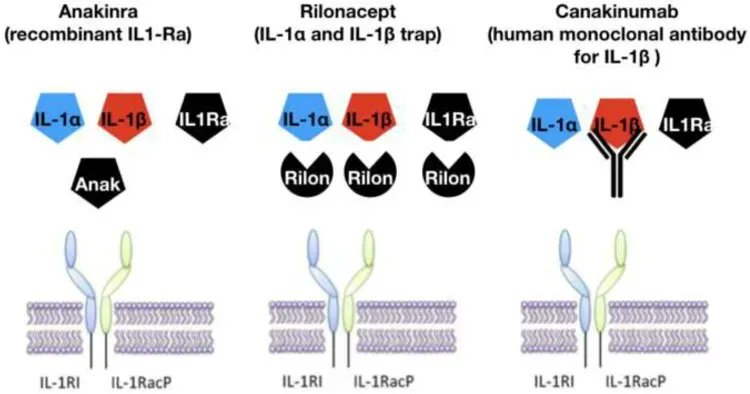

Dr. John Cush RheumNow
3 years 6 months ago
USPSTF recommends clinicians prescribe Statins for the primary CVD prevention in adults (40-75 yrs) w/ 1 or more CVD risk factors (ie, dyslipidemia, DM, HTN, smoking) & 10-year CVD risk of 10% or greater. Inflammatory dz is considered a CV risk enhancer https://t.co/1Opblbiz7B https://t.co/xSw2uPUoKK
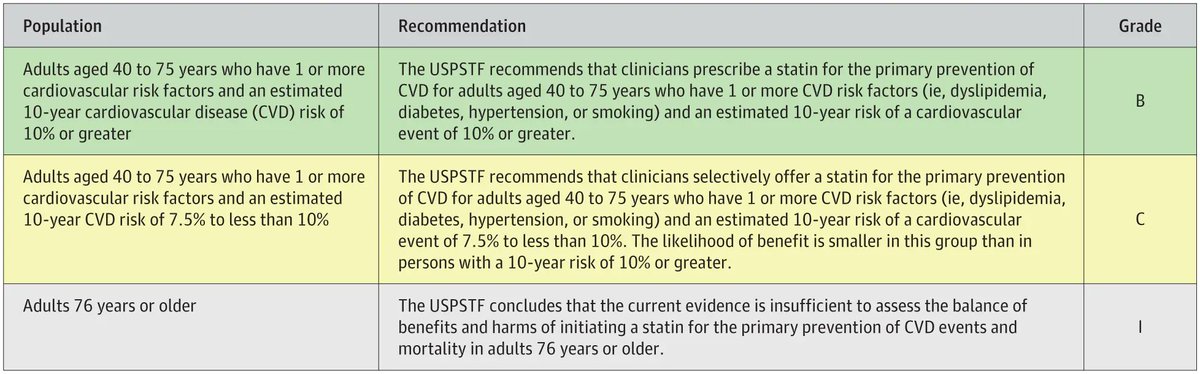

Dr. John Cush RheumNow
3 years 6 months ago
Review of steroids & immunosuppressants on cancer outcomes in checkpoint inhibitor therapy
- Steroid use "for irAEs may not have a large deleterious effect on overall survival"
- DMARDs for irAE "a major drawback in the delay in the onset of response"
https://t.co/8o8JBMpqML https://t.co/bWuLT3zYPm
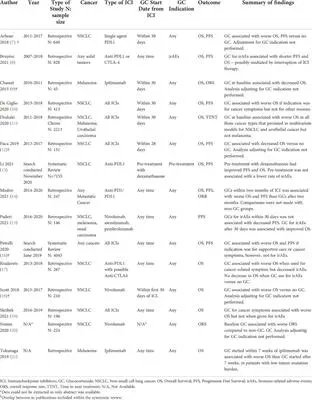

Dr. John Cush RheumNow
3 years 6 months ago
MIRROR study lead to pegloticase (PEG) label change - adding MTX as Rx to reduced anti-PEG Abs. Based on 14 refractory gout pts given MTX 15mg/wk (w/ folate) and then PEG IV: 3 nonresponders, 1 dropped out, 10 responded/stayed on Rx x 52 wks w/ less ADAbs https://t.co/BZwY7DpmIb https://t.co/gNhhuhmTG5
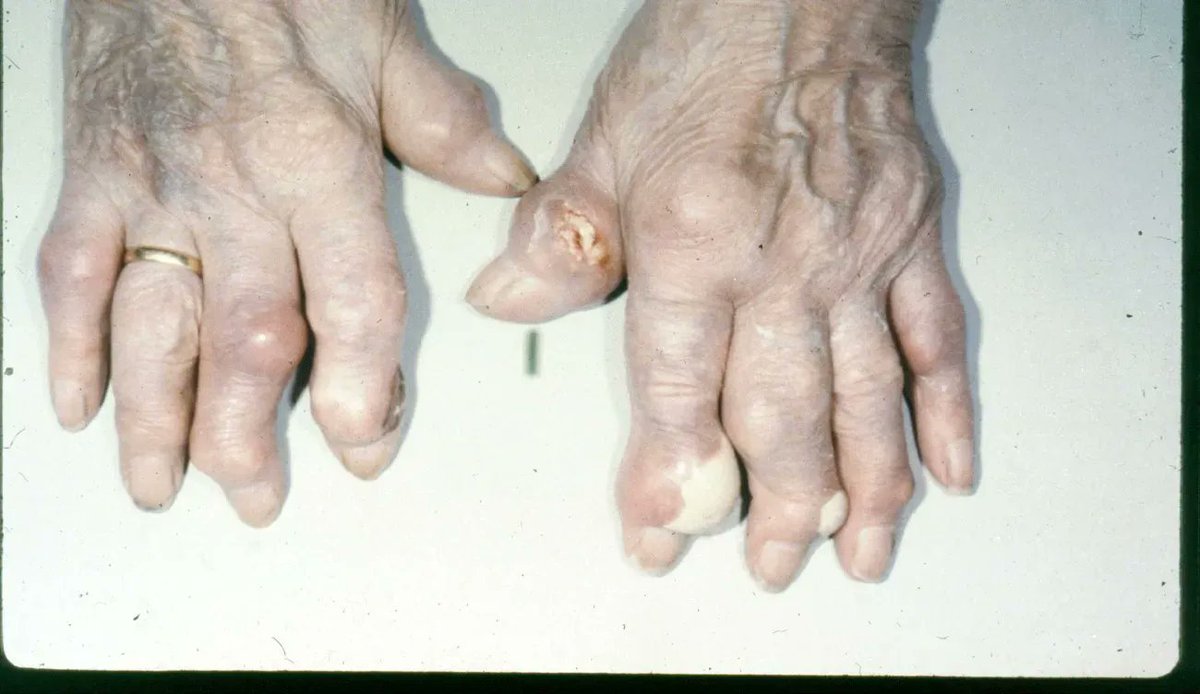

Dr. John Cush RheumNow
3 years 6 months ago
Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.
https://t.co/ZaWnjxiR3W https://t.co/8CElIxWk1v https://t.co/ZVayc3Qt1r


Dr. John Cush RheumNow
3 years 6 months ago
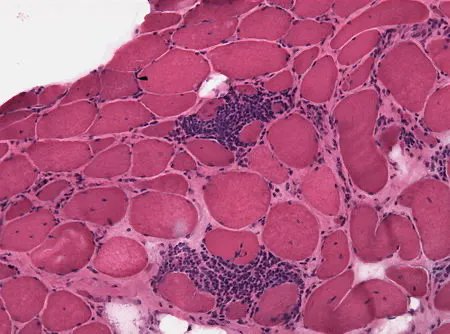
EULAR/ACR Classification in MDA5+ Myositis Patients
A current analysis suggests that nearly 30% of MDA5 IIM patients may be missed by the 2017 EULAR/ACR classification criteria for IIM.
https://t.co/rS25rL5fGO https://t.co/Yw7sGmPW5G

Dr. John Cush RheumNow
3 years 6 months ago
Drugs causing Scleroderma (DASSc) - Anticancer rx made up 42% DASSc & 62.3% case reports. Included: taxane-based agents, bleomycin, vinblastine, imatinib, dacarbazine, pembrolizumab pemetrexed; also HRT romiplostim & eculizumab https://t.co/szQmNPO0eu https://t.co/z4tdP4I1kc

Dr. John Cush RheumNow
3 years 6 months ago
A Rheum & Derm Conversation on Treating PsA and Moderate-to-Severe PsO
Sponsored by Lilly USA
Listen to Philip Mease and April Armstrong discuss a multidisciplinary approach and importantconsiderations when treating patients with joint and
skin concerns.
https://t.co/lTfz2kUr51 https://t.co/jVp8Uk0uH5


Dr. John Cush RheumNow
3 years 6 months ago
Longitudinal study of 411 adult fibromyalgia pts showed that 73.2% had depression at enrollment. Higher depression scores at baseline predicted poorer functional outcomes. Effectively treated depression resulted in improved functioning at follow-up. https://t.co/D43r4Z5llU https://t.co/a9EgyJLHza


Dr. John Cush RheumNow
3 years 6 months ago
Head Scratcher? Study of UK Biobank cohort (n=380,380) shows those taking Rx Folate had increased risk of COVID-19 Dx (OR 1.51; 1.42–1.61) and Death (OR 2.64; 2.15–3.24). MTX use (+/- folate) had no increase in COVID or COVID death(1.07;0.57–1.98) https://t.co/MLE1sL7KG2 https://t.co/adoLO9qLON




























 Poster Hall
Poster Hall