
Dr. John Cush RheumNow
3 years 6 months ago
FDA Approves BA.4 and BA.5 Omicron COVID Booster Vaccines
Today the FDA authorized the updated Omicron subvariant (BA.4 and BA.5) COVID-19 booster shots manufactured by Pfizer and Moderna; with an anticipated ship/start date of early September 2022.
https://t.co/VzQBFgvAqu https://t.co/r0pQuoDVcn
Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.com.
While hypertension and arthritis are very common disorders, the association between them is unclear. Analysis of NHANES data suggests that both rheumatoid arthritis (RA) and osteoarthritis (OA) are strongly associated with hypertension (HTN).
The NHANES (National Health and Nutrition Examination Survey) of non-pregnant adult, aged ≥ 20 years examined data 48,372 eligible participants between 1999–2018.
Not all patients with periodic fevers fit neatly into diagnostic categories. Some can be diagnosed as Still’s disease (based on criteria) while others can be classified as autoinflammatory diseases (AID) and some may be unclassifiable, clinically or genetically.

Dr. John Cush RheumNow
3 years 6 months ago
Study of 497 pts referred by general practitioners to rheumatologist (2018-19) showed:
- GP Dx changed by Rheum in 58%
- Low complexity Dx (FM, OA) acct for 50% referrals
- 46% of referrals inappropriate
- GP correlations w/ Rheum Dx poor (κ < 0.5) https://t.co/q592iHQZbq https://t.co/XyPdtpHQGK

Dr. John Cush RheumNow
3 years 6 months ago
Recent review of AOSD suggests: - AOSD & SJIA are the same - Classify dz as systemic or articular - #MAS occurs in up to 23% - Yamaguchi & Fautrel criteria are widely used - Cytokines important: IL-1, IL-6, IL-18, IL-37 - only Canakinumab is FDA-approved https://t.co/4ERA56Wqcb https://t.co/FkIWxIrwxX

Dr. John Cush RheumNow
3 years 6 months ago
NICE Guidelines on Gout Diagnosis and Management
NICE (UK) has systematically reviewed current medical evidence and delivered a set of recommendations with consideration of cost effectiveness.
https://t.co/ckDrfVAynN https://t.co/jzBviwpxGL
The diagnosis of idiopathic inflammatory myopathies (IIMs) can be informed by the 2017 EULAR/ACR classification criteria, but their utility in patients with clinically amyopathic dermatomyositis (CADM) and anti–melanoma differentiation–associated protein 5 (anti–MDA-5)–positive IIM is less certain.

Dr. John Cush RheumNow
3 years 6 months ago
Risk of TNFi-related psoriasis in 5088 TNFi Rx children w/ IBD, JIA, or CNO. Compared to non-TNFi exposed, kids on TNFi had an increased PSO risk w/ ADA (IRR 2.70; 1.53-4.75), infliximab (2.34) or etanercept (2.21) & not incr w/ DMARD use https://t.co/MyjLfeoChq https://t.co/A3JJUA1AaM

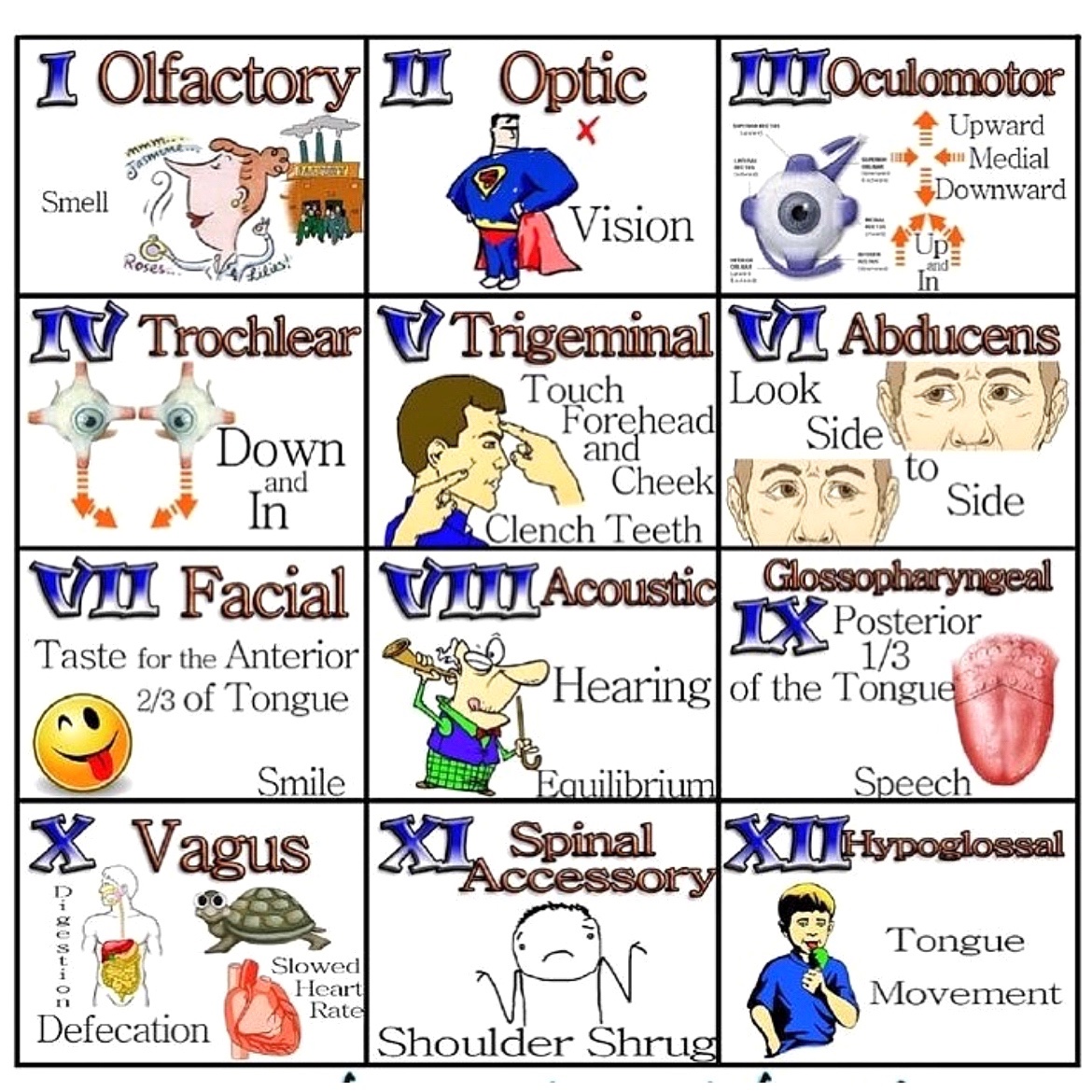
Brown Journal of Hospital Medicine BrownJHM
3 years 6 months ago
Cranial nerves exam (unknown source) #BJHM #Neurotwitter #Medtwitter #MedEd #medstudents https://t.co/NnUx6tGERn

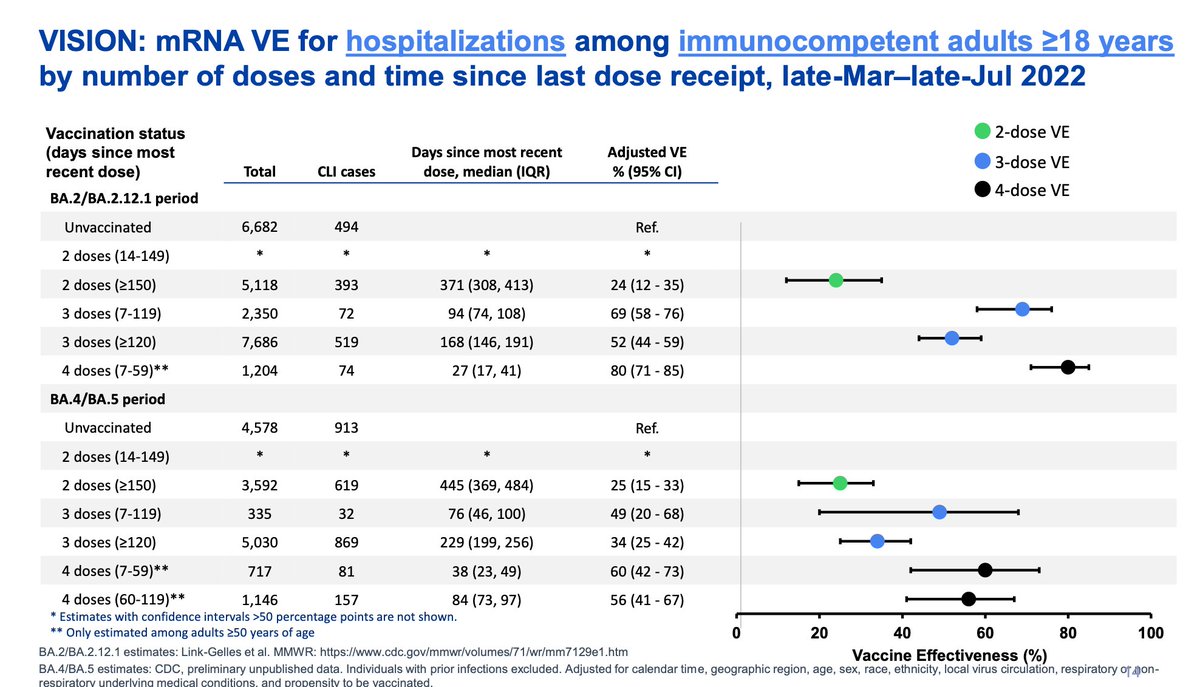
Eric Topol EricTopol
3 years 6 months ago
New data at today's CDC/ACIP meeting on vaccine effectiveness vs BA.5 wave hospitalizations, all adults
https://t.co/64b9g7HW1h
Less protection for both 3rd and 4th dose vaccines compared with the prior wave (BA.2, BA.2.12) https://t.co/JTichjw2I3

Dr. John Cush RheumNow
3 years 6 months ago
Calling All Rheumatology Fellows! Join the ACR Fellows-in-Training Subcommittee https://t.co/FSyMjVieAD

Dr. John Cush RheumNow
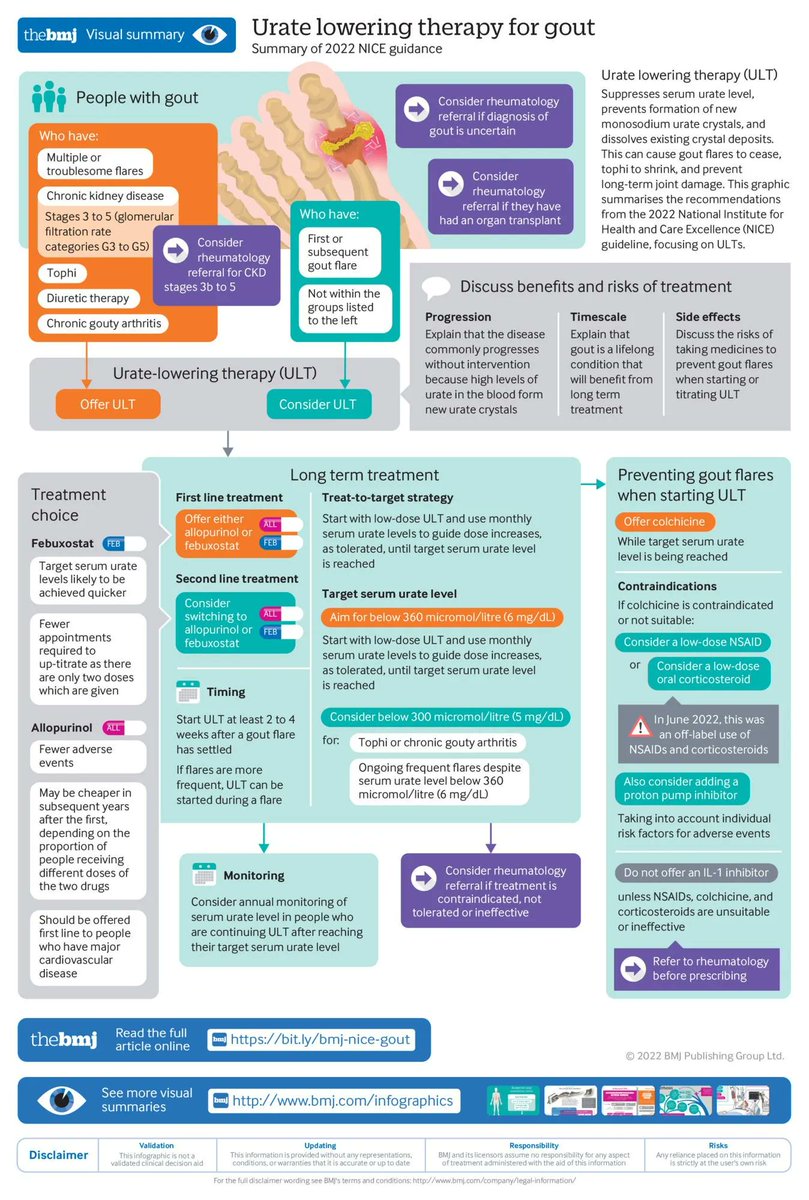
3 years 6 months ago
Gout: diagnosis and management—summary of NICE guidance https://t.co/zOdPzGtqg7 https://t.co/N7CJ23vLND

Dr. John Cush RheumNow
3 years 6 months ago
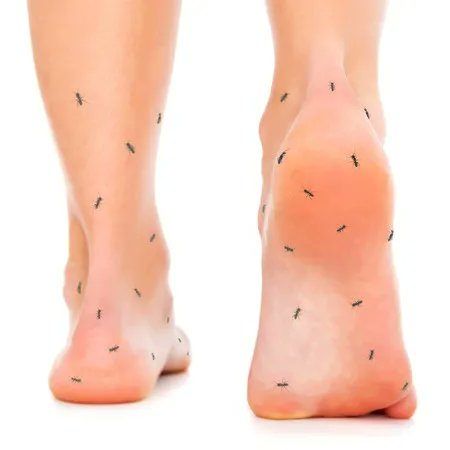
OPTION-DM study of 140 diabetic neuropathy pts Rx for 16 weeks w/ one agent (& if no response) an add-on agent. At wk 16, all 3 regimens lowered nerve pain score from 6.6 to 3.3 w/ no difference between Elavil+Lyrica, Lyrica+Elavil, o Cymbalta+Lyrica) https://t.co/rijJyq1DYL https://t.co/NwHF6xuF0j






























 Poster Hall
Poster Hall