Drug Safety
An integrated analysis of two pivotal trials of voclosporin, a calcineurin inhibitor, in lupus nephritis patients saw significant improvement in complete renal responses (CRR) at one year.

Dr. John Cush RheumNow
3 years 5 months ago
B12 or Placebo effect? Open label study of B12 (1000mcg/d) in 29 fibromyalgia pts showed after 50d, significant reductions in FM Impact Quest. (FIQR; 49.8 to 40.00; p< 0.01), Function, Sxs, anxiety scores all signif improved. https://t.co/MtJWy5xlz9 https://t.co/kzvA1wd3zb

Dr. John Cush RheumNow
3 years 5 months ago
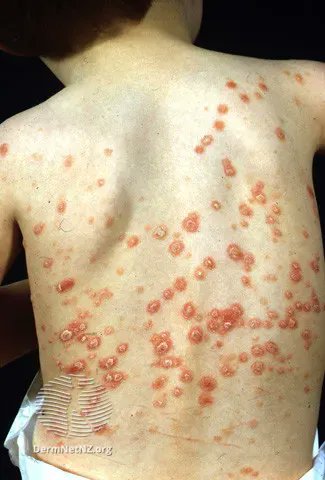
Risk of TNFi-related psoriasis in 5088 TNFi Rx children w/ IBD, JIA, or CNO. Compared to non-TNFi exposed, kids on TNFi had an increased PSO risk w/ ADA (IRR 2.70; 1.53-4.75), infliximab (2.34) or etanercept (2.21) & not incr w/ DMARD use https://t.co/Y1Vn9kjk5N https://t.co/5CCxa5kMcU

Dr. John Cush RheumNow
3 years 5 months ago
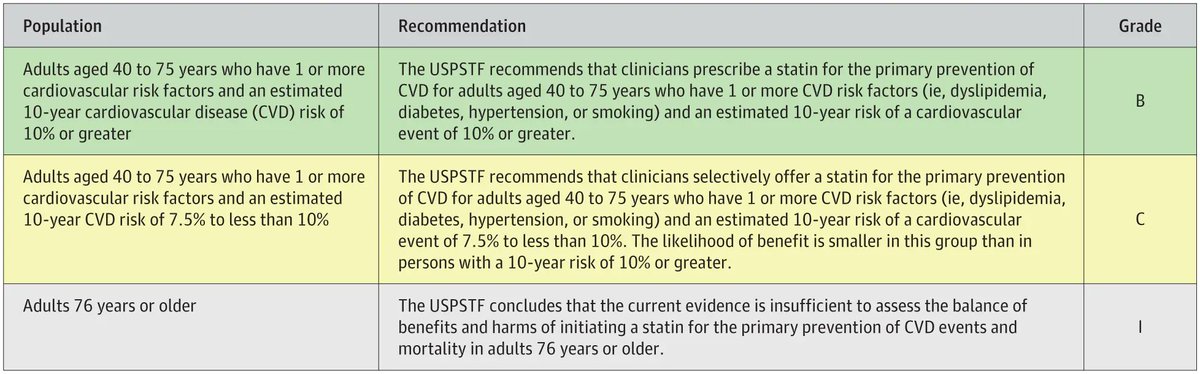
USPSTF recommends clinicians prescribe Statins for the primary CVD prevention in adults (40-75 yrs) w/ 1 or more CVD risk factors (ie, dyslipidemia, DM, HTN, smoking) & 10-year CVD risk of 10% or greater. Inflammatory dz is considered a CV risk enhancer https://t.co/1Opblbiz7B https://t.co/xSw2uPUoKK

Dr. John Cush RheumNow
3 years 5 months ago
Review of steroids & immunosuppressants on cancer outcomes in checkpoint inhibitor therapy
- Steroid use "for irAEs may not have a large deleterious effect on overall survival"
- DMARDs for irAE "a major drawback in the delay in the onset of response"
https://t.co/8o8JBMpqML https://t.co/bWuLT3zYPm
Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.com.

Ian N Bruce Lupusdoc
3 years 6 months ago
In a nutshell, reducing glucocorticoids in #SLE patients is associated with a number of important clinical benefits and may impact risk of other comorbidities such as BP and infection risk. https://t.co/YBoIU6aK8p
Today the FDA authorized the updated Omicron subvariant (BA.4 and BA.5) COVID-19 booster shots manufactured by Pfizer and Moderna; with an anticipated ship/start date of early September 2022. The BA.5 subvariant accounts for more than 88% of U.S. infections.

Dr. John Cush RheumNow
3 years 6 months ago
Statin Myalgias? Lancet metanalysis of 19 PCTs, >80K pts, @ 1yr, Statin caused a 7% increase in muscle pain or weakness (1·07; 1·04–1·10); thus 1 in 15 had myalgia due to statin (1/10 w/ high dose). After year 1, no signif excess in statin related myalgia https://t.co/6cRJQMymzM https://t.co/RTH56J4FD1

NICE (UK) has systematically reviewed current medical evidence and delivered a set of recommendations with consideration of cost effectiveness.
Data from Data FORWARD (The National Databank for Rheumatic Diseases) reveals that nearly two-thirds of rheumatoid arthritis (RA) patients have self-reported sleep problems.
The FORWARD study, a registry that includes over 4200 RA patients), collects data on obstructive sleep apnea (OSA), restless legs syndrome (RLS), and short sleep (SS) problems. SS was based on self-reported average sleep time (<6 hours).

Dr. John Cush RheumNow
3 years 6 months ago
Review of VTE risk with JAK inhibitors
- Tofacitinib: 10mg or 5 mg bid - PE rates 0.5 & 0.3/100PY; higher w/ risk factors, VTE 0.35/100PY
- Baricitinib: IR of DVT/PE was 0.5/100PY
- Filgotinib: 200mg VTEs 0.2/100PY
Most VTE occured in LT extension of RCT https://t.co/xvf0kQUMel https://t.co/Bj6fmkktP7
Dr. Jack Cush delivers this weeks rheumatology "weather report" with the best and least of news and journal articles from the past week on RheumNow.com
“Climate is what we expect, weather is what we get.” – Mark Twain

Dr. John Cush RheumNow
3 years 6 months ago
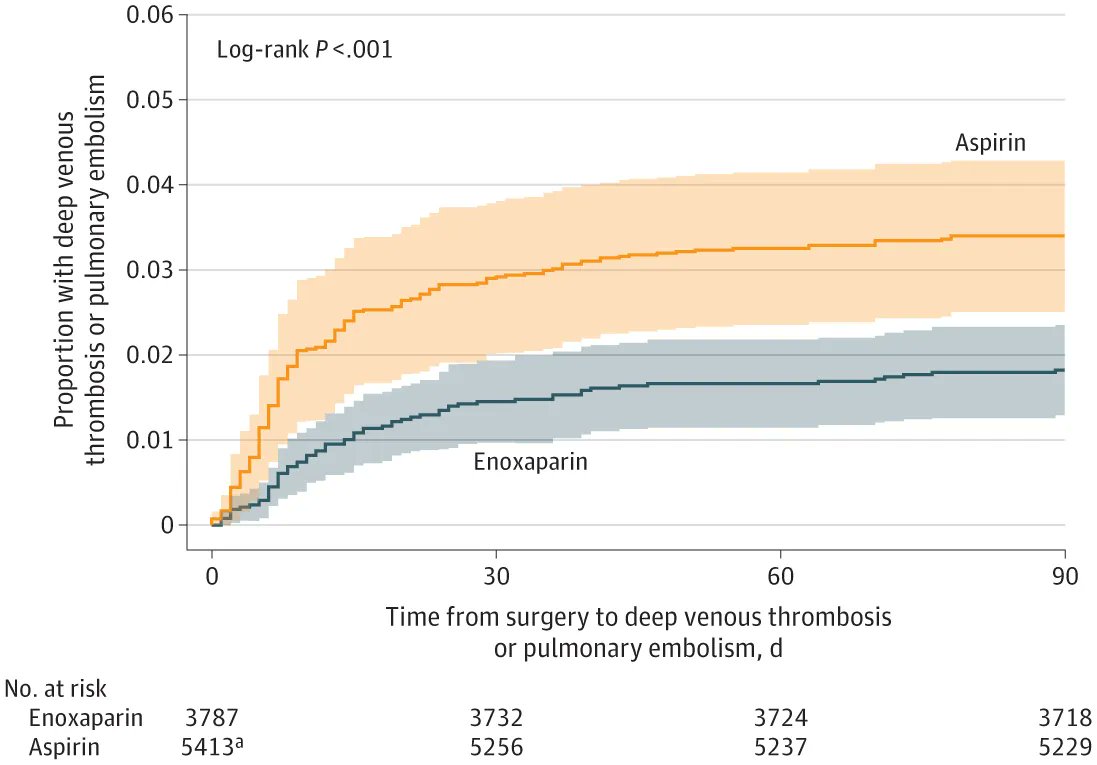
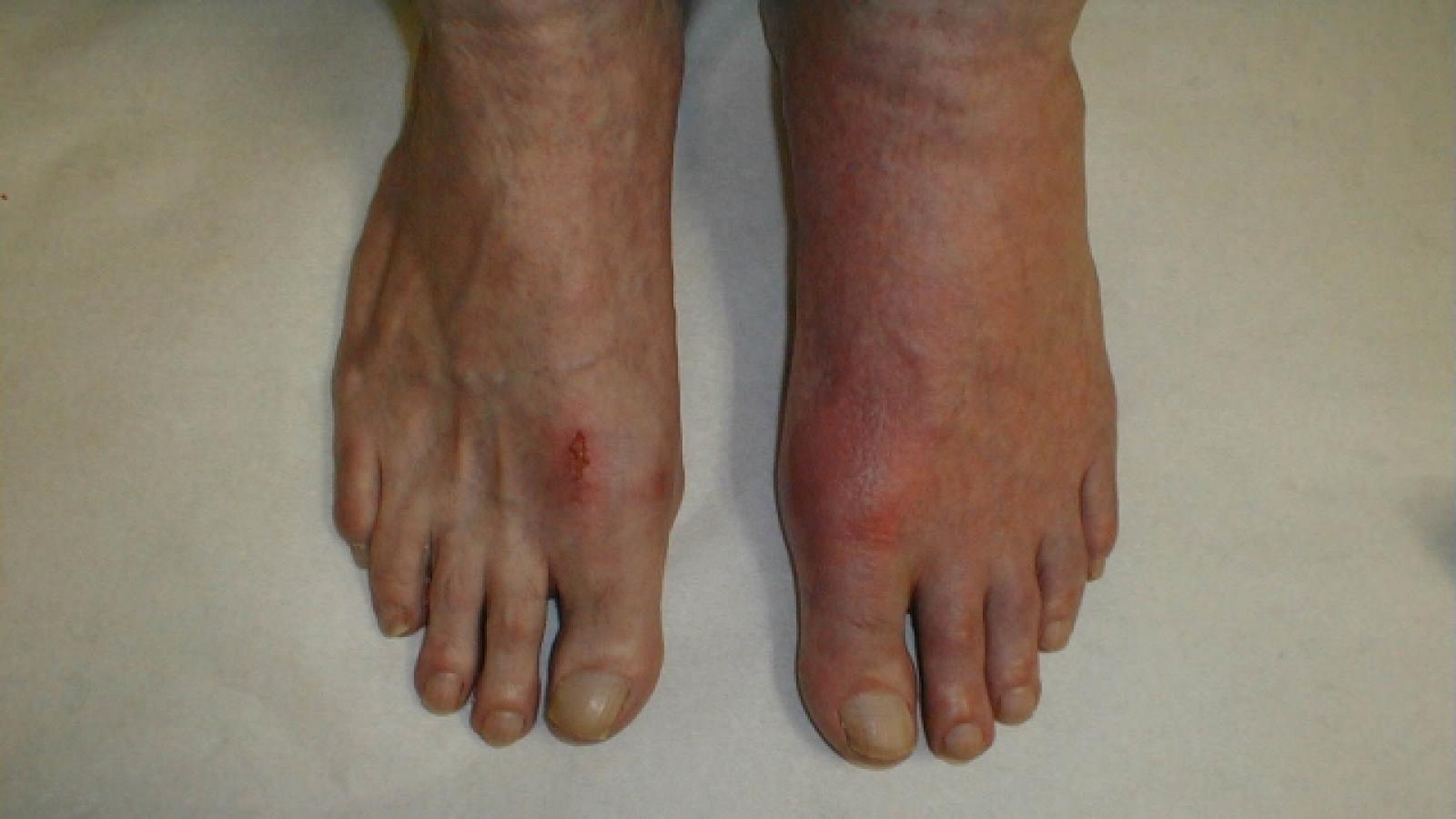
Study of 9611 pts w/ hip or knee arthroplasty for #OA tested aspirin (100 mg/d) vs enoxaparin (40 mg/d) (x35d w/THA; 14d after TKA). Enrollment prematurely halted as VTE rate was 3.45% w/ ASA and 1.8% w/ enoxaparin (superior to ASA: P= .007) https://t.co/jvuely8L56 https://t.co/O1TxCjaSPU
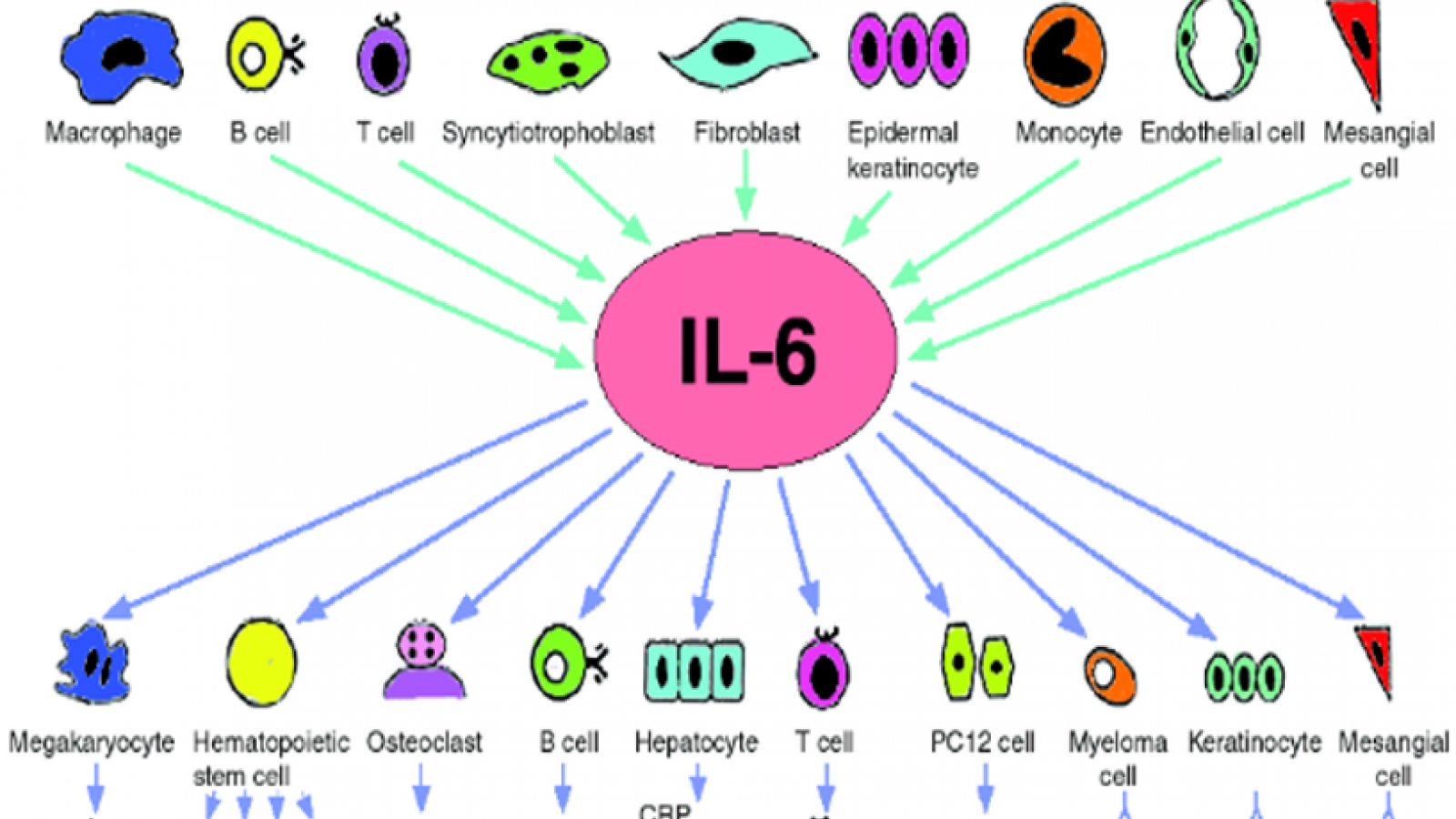
This week's NEJM has published the efficacy results of a large phase 3 trial of olokizumab, a humanized monoclonal antibody that directly targets IL-6 in patients with rheumatoid arthritis.
This is in contrast to two other marketed IL-6 inhibitors (sarilumab, tocilizumab) that bind to the IL-6 receptor.











 Poster Hall
Poster Hall