Drug Safety
The big news this week: the approval of deucravacitinib (a new class of drug?) for psoriasis; the 2022 ACR guidance on glucocorticoid-induced osteoporosis; a national poll of older adults over the age of 50 who claimed self-reported or doctor-diagnosed arthritis; and much more. Let's review these and other news, journal reports and announcements from this past week.
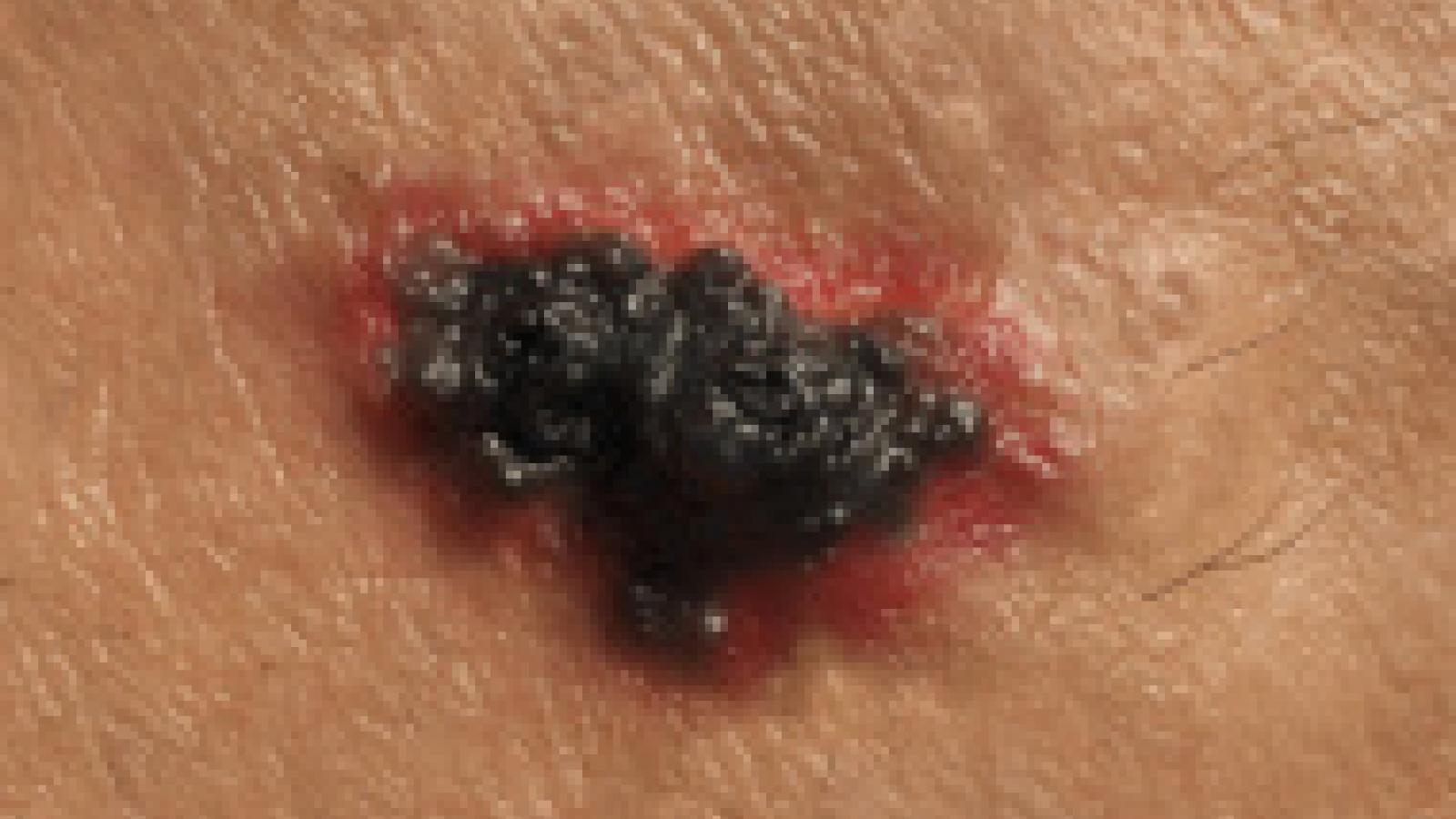
A systematic review suggests that low-dose methotrexate (MTX) use is associated with an increased melanoma risk, but the absolute risk increase could be considered negligible.
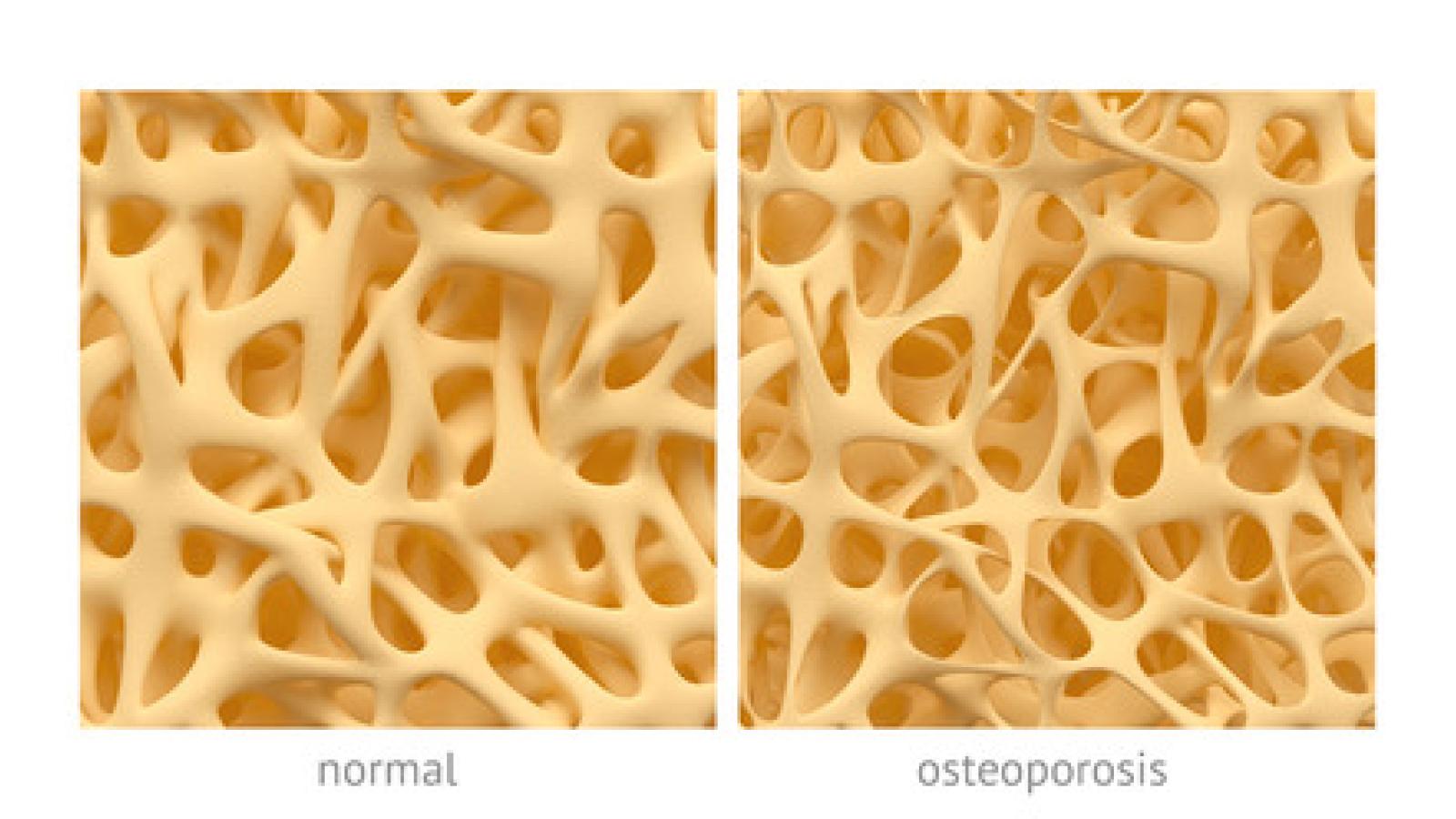
The ACR has updated this guideline and includes recommendations on abaloparatide (PTHrP) and romosozumab, which are newly available since the ACR’s 2017 GIOP guideline.
Two large RA registries have shown that pregnancy outcomes in rheumatoid arthritis (RA) patients is more related to RA disease activity rather than treatments use to control RA.
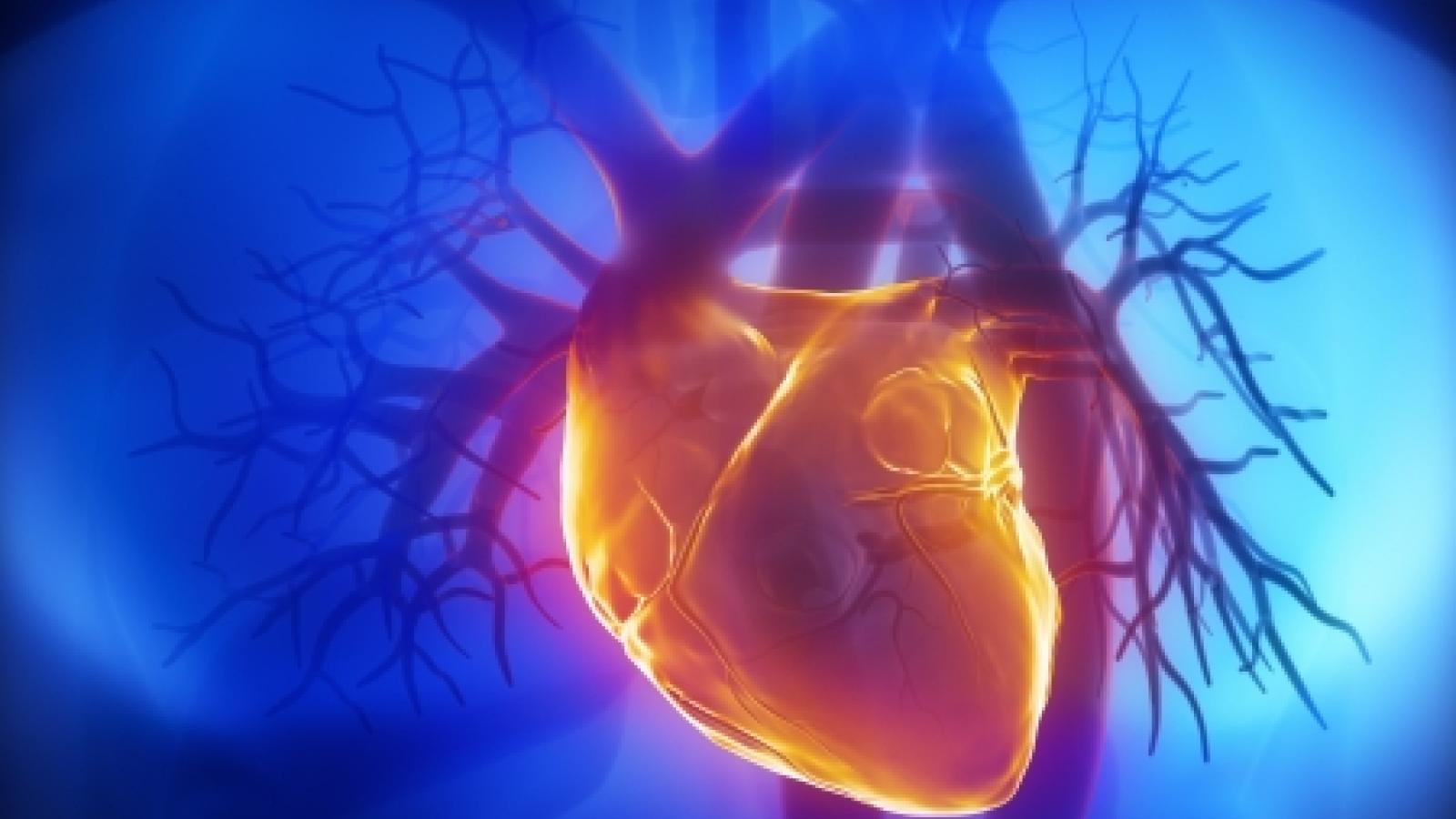
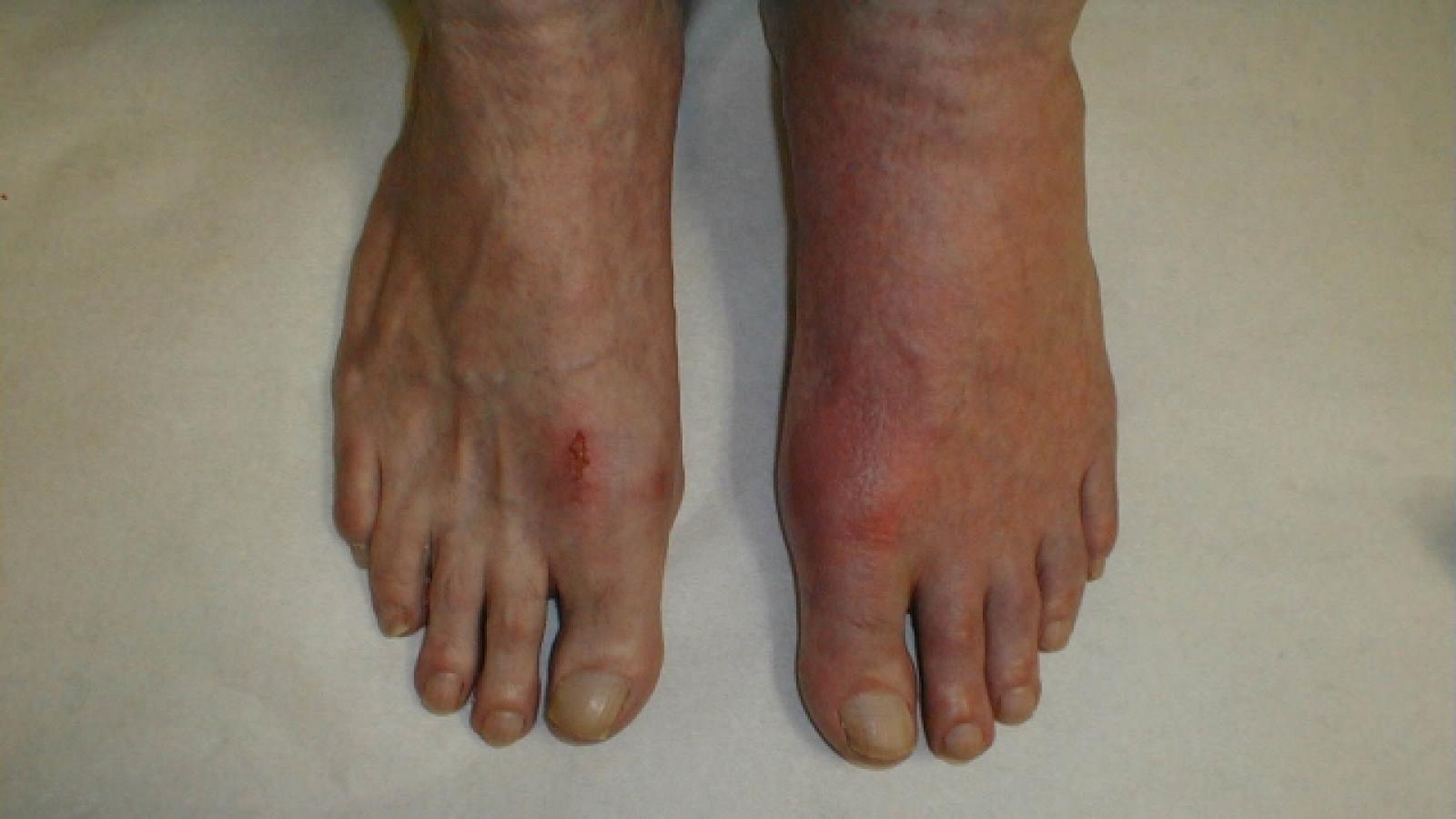
Incidence of acute coronary syndrome (ACS) was significantly higher among gout patients than in the general Swedish population, researchers found, even when data were adjusted for common comorbidities.
Forbes reports that a recent Merritt Hawkins survey shows that patients are waiting an average of 26 days for a scheduled appointment with a doctor.
This is based on a 15 U.S. city survey of more than 1,000 physician offices - including family medicine, dermatology, obstetrics/gynecology, orthopedic surgery and cardiology practices.
The average wait time is up 8% from 24.1 days in 2017 (It was 21 days in 2004).
Deucravacitinib (Sotyktu), a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, is the only approved TYK2 inhibitor worldwide and the first innovation in oral treatment for moderate-to-severe plaque psoriasis in nearly 10 years.
Can we predict the bad outcomes? Like when ITP evolves into SLE; or when psoriasis will develop arthritis; or if Sjogren's will develop lymphoma? Let's dive in and review these journal reports and this past week's news from RheumNow.com.
Rheumatoid arthritis patients with existing interstitial lung disease (ILD) had less decline in lung function when receiving the antifibrotic agent pirfenidone (Esbriet) relative to placebo in a randomized trial, researchers reported here and in a simultaneous journal publication.
Among U.S. veterans receiving hydroxychloroquine (HCQ) as long-term therapy for rheumatoid arthritis, development of long QT syndrome was rare and not markedly more common compared with similar patients treated with other agents, researchers said.
An integrated analysis of two pivotal trials of voclosporin, a calcineurin inhibitor, in lupus nephritis patients saw significant improvement in complete renal responses (CRR) at one year.
Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.com.
Today the FDA authorized the updated Omicron subvariant (BA.4 and BA.5) COVID-19 booster shots manufactured by Pfizer and Moderna; with an anticipated ship/start date of early September 2022. The BA.5 subvariant accounts for more than 88% of U.S. infections.
NICE (UK) has systematically reviewed current medical evidence and delivered a set of recommendations with consideration of cost effectiveness.
Data from Data FORWARD (The National Databank for Rheumatic Diseases) reveals that nearly two-thirds of rheumatoid arthritis (RA) patients have self-reported sleep problems.
The FORWARD study, a registry that includes over 4200 RA patients), collects data on obstructive sleep apnea (OSA), restless legs syndrome (RLS), and short sleep (SS) problems. SS was based on self-reported average sleep time (<6 hours).













 Poster Hall
Poster Hall