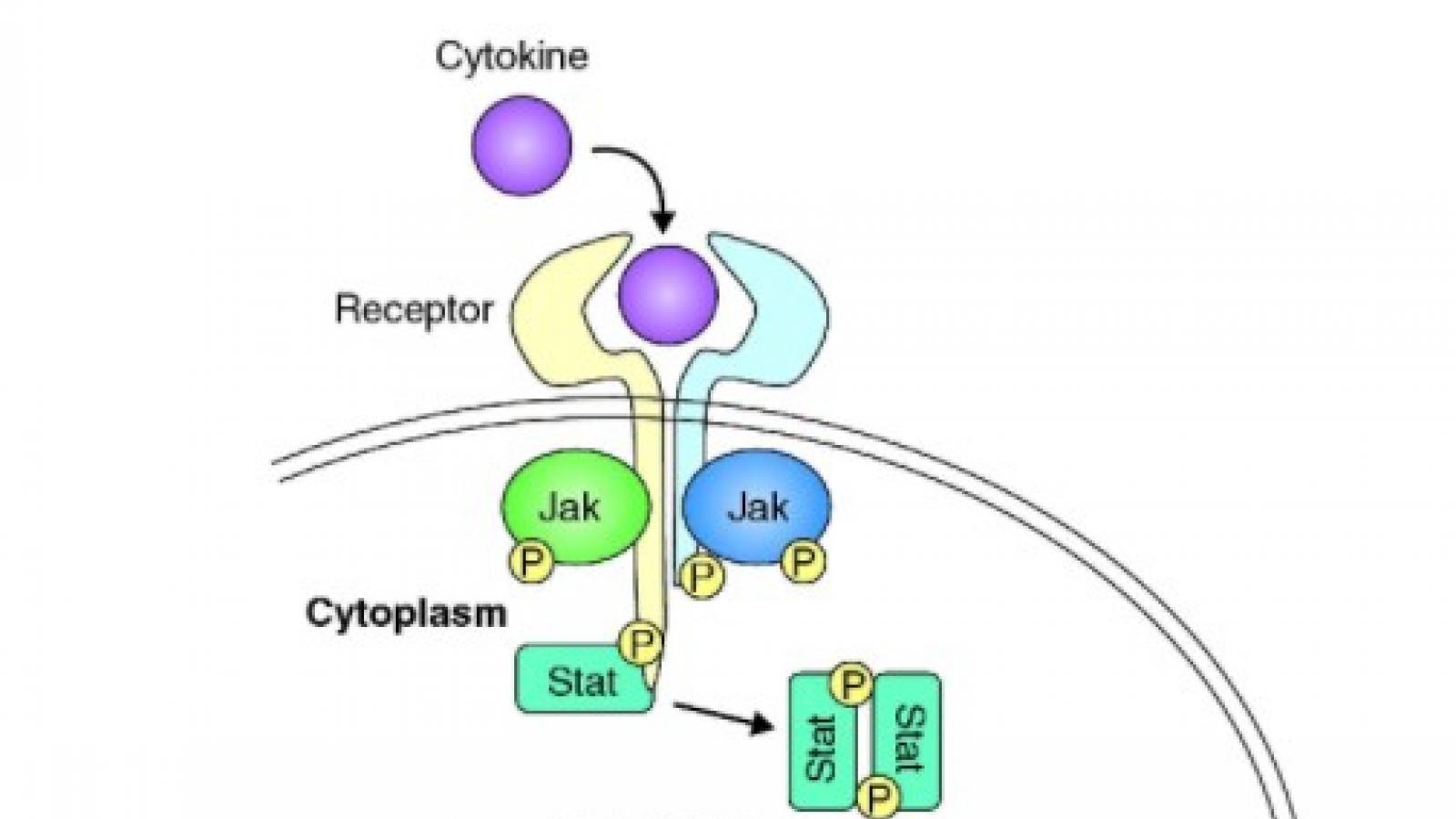
IL-17
With the recent publication of the third iteration of the GRAPPA Psoriatic Arthritis (PsA) treatment recommendations, it seems to be an auspicious time to reflect on some key considerations that arose during the development of the recommendations, as well as to look towards what the future may hold.

Dr. John Cush RheumNow
3 years 7 months ago
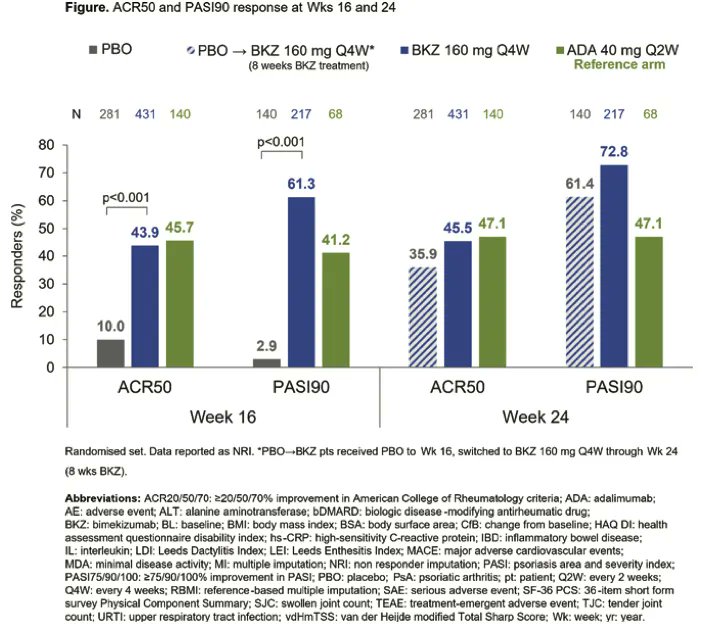
Bimekizumab: IL-17A and F inhibitor for tx of PsA - BE OPTIMAL trial reached primary endpoint: ACR50 44% vs. placebo
Efficacy as early as 2 weeks
No MACE, uveitis, IBD, deaths
@RheumNow #EULAR2022 ABST#LB0001 cc:@doctorRBC : https://t.co/wgeBsfdQcL
For 2022, let's look our top 10 list of advances, game-changers, worries and those better medical practices that evolved during 2021.
With their publication in June 2022 (1), the 3rd iteration of the Group for Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Treatment recommendations for Psoriatic Arthritis (PsA) may have set a record or sorts.

Dr. John Cush RheumNow
3 years 7 months ago
EMA has approved Secukinumab for the expanded approvals for use in pediatric patients with enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA) . Approvals based on the JUNIPERA trial, showing reduced the risk of flare & Dz activity w/ SEC https://t.co/6pWc6nEUd9 https://t.co/gGGyzEEh0u


Dr. John Cush RheumNow
3 years 7 months ago
Inferior IL-17 Inhibitor Responses in Psoriatic Women
Pooled data from two large (IXE trials show that male patients had greater clinical responses than did female patients with PsA are largely unexplored. Reasons for this is unexplained.
https://t.co/OAu6gCweDt https://t.co/lsM6hZUwNQ
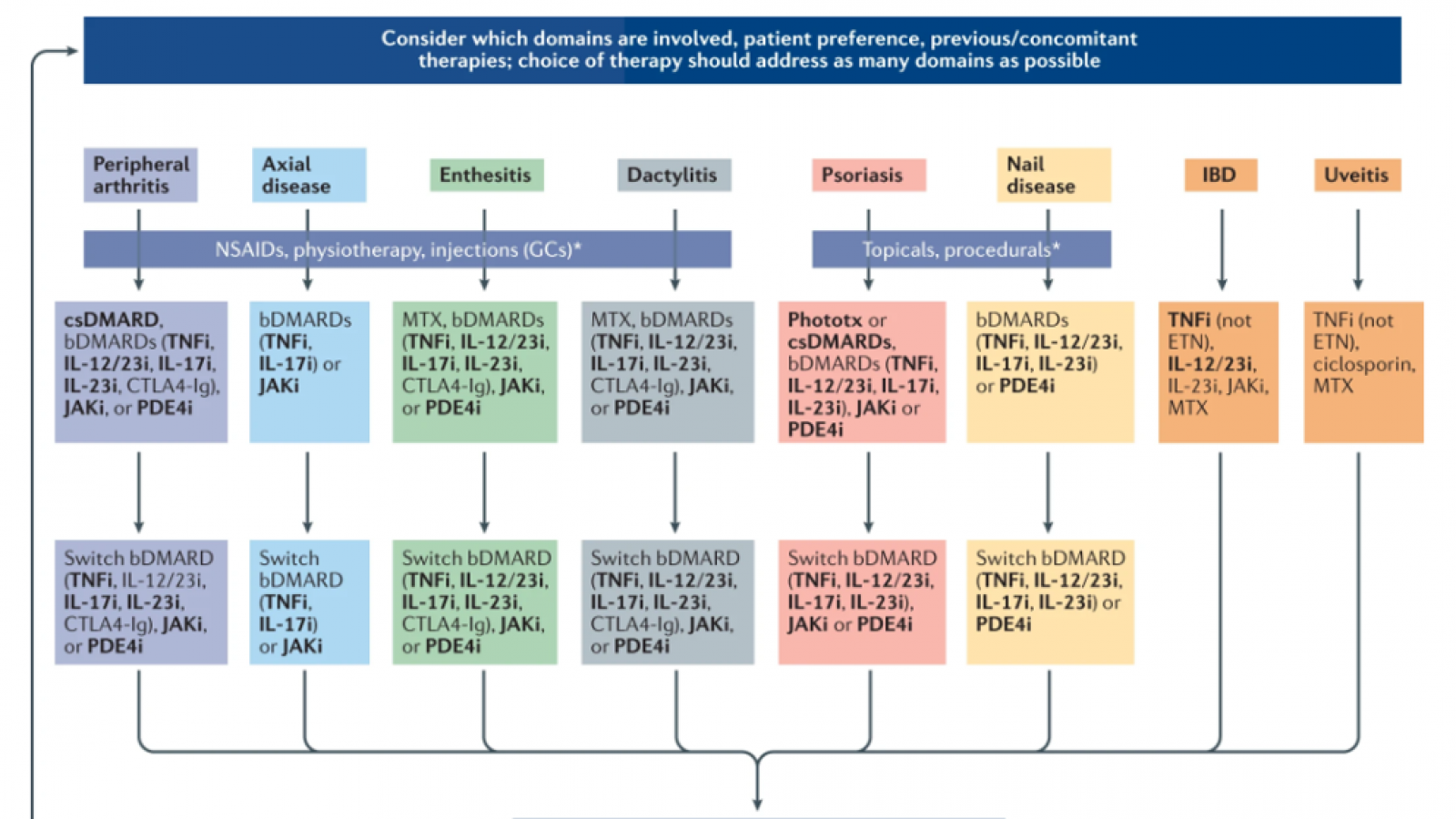
The GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) treatment recommendations have been updated and the 2021 evidence-based guidance is rich in treatment recommendations based on the key disease "domains" - peripheral arthritis, axial disease, enthesitis, dactylitis, skin and nail psoriasis; with new PsA related domains uveitis and inflammatory bowel disease.
Pooled data from two large ixekizumab (IXE) trials show that male patients had greater clinical responses than did female patients with psoriatic arthritis are largely unexplored. Reasons for this differential treatment responses to an interleukin-17A inhibitor (IXE) between male and female patients is unexplained.

Dr. John Cush RheumNow
3 years 7 months ago
Treatment of pyoderma gangrenosum is clinically empiric. Full read ref w/ Graded Rx Recommendations:
- 1st line: Steriods or Cyclosporin
- Consider biologics: TNFi, IL-1, IL-12/23, IL-17, IL-23
- Others: MTX, MMF, AZA, Dap, colchic, thalidomide, IVIG https://t.co/uMJyfFzsRB https://t.co/vw4VqbjZWh

A pooled analyses of data from eight, phase 2 and 3 randomized clinical trials shows bimekizumab, a dual IL-17A/F inhibitor, to be effective in plaque psoriasis and was well tolerated aside from an increased incidence of mild to moderate oral candidiasis.
Pyoderma gangrenosum (PG) is rare, but often associated with different forms of arthritis, in particular rheumatoid arthritis and inflammatory bowel diseases.
There are still questions surrounding COVID-19, and some common questions I receive from patients revolve around what to do with their current DMARDs or should they even start treatment during this pandemic. Two studies focused on this question.














 Poster Hall
Poster Hall