All News
Which Biologics are Best in Psoriasis
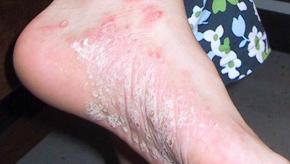
A metanalysis of phase II, III and IV trials in moderate to severe plaque psoriasis suggests comparative efficacy biologic treatments, but that that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were shown to have the past skin (PASI) response rates.
Read ArticleAspirin after Hip or Knee Arthoplasty
JAMA Internal Medicine has reported that use of low dose aspirin for venous thromboembolism (VTE) prophylaxis after total hip and knee replacement is equal in efficacy to other anticoagulants.
Read ArticleStart with Anti-TNF in RA? Not So Fast
The suggestion to hit rheumatoid arthritis (RA) early and hard with biologic therapies itself took a hit in a new study.
Read ArticleLimited Advantage to Very Early vs. Delayed Etanercept in RA
The VEDERA study sought to confirm whether the very early introduction of first-line etanercept+methotrexate (ETN+MTX) was superior to treat-to-target MTX (MTX-TT) in patients with early RA.
Read ArticleEULAR Recommendations on Sjögren’s Syndrome
The European League Against Rheumatism (EULAR) has established an international collaborative group (EULAR SS Task Force) to develop the first EULAR evidence and consensus-based recommendations for the management of patients with Sjogens syndrome (SjS).
Read ArticleHalf of Opioids Rx Come from 1% of MDs
The BMJ reports that while most US providers are cautious in their prescribing, half of opioid prescriptions are written by 1% of providers.
Between 2003 and 2017, there was an annual average of 669495 providers prescribing 8.9 million opioid prescriptions.
Read ArticleRheumNow Podcast- 2019 EULAR RA Guidelines (1.31.20)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Read ArticleHormone Therapy for Postmenopausal Women
The NEJM weighs in on the problem of post-menopausal osteoporosis (OP) and tackles the use of hormonal therapy.
The decline in estrogen after menopause may increase risks for osteoporosis, cardiovascular disease, and cognitive decline. The use of hormone replacement therapy (HRT) to obviate these issues may be primarily driven by hot flashes in postmenopausal women.1
Who may benefit from hormone therapy among postmenopausal women?
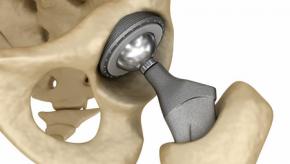
Knee Replacements Last 25 Years
UK registry reports that greater than 80% of total knee replacements can last for 25 years.
The outcomes regarding the duration and durability of knee arthroplasties is sketchy, with many orthopedists projecting a 15 to 20 year survivial. Hence the need for an appraisal of the data.
Read ArticleRheumatoid Arthritis Antibodies Linked With COPD
Women who were seropositive for anti-citrullinated protein antibodies (ACPA) and ultimately went on to develop rheumatoid arthritis (RA) were also at increased risk for being diagnosed with chronic obstructive pulmonary disease (COPD), analysis of data from the Nurses' Health Study showed.
Read ArticleBook Review: “Great Health Care Value: Chronic Diseases, Practice Teams and Population Management”
The US healthcare market has evolved into an incredibly expensive system that often does not deliver good medical outcomes. While most of us know these problems exist, we rarely have up-to-date data or can offer alternatives to the way we manage care, especially to the chronically ill who consume much of our health care dollars. In their book, authors Tim Harrington, MD and Andrew Johnson, MS, MBA offer insights, evidence and experience on how we may do our part to improve the management of chronic rheumatic/orthopedic issues.

Dr. John Cush RheumNow ( View Tweet)
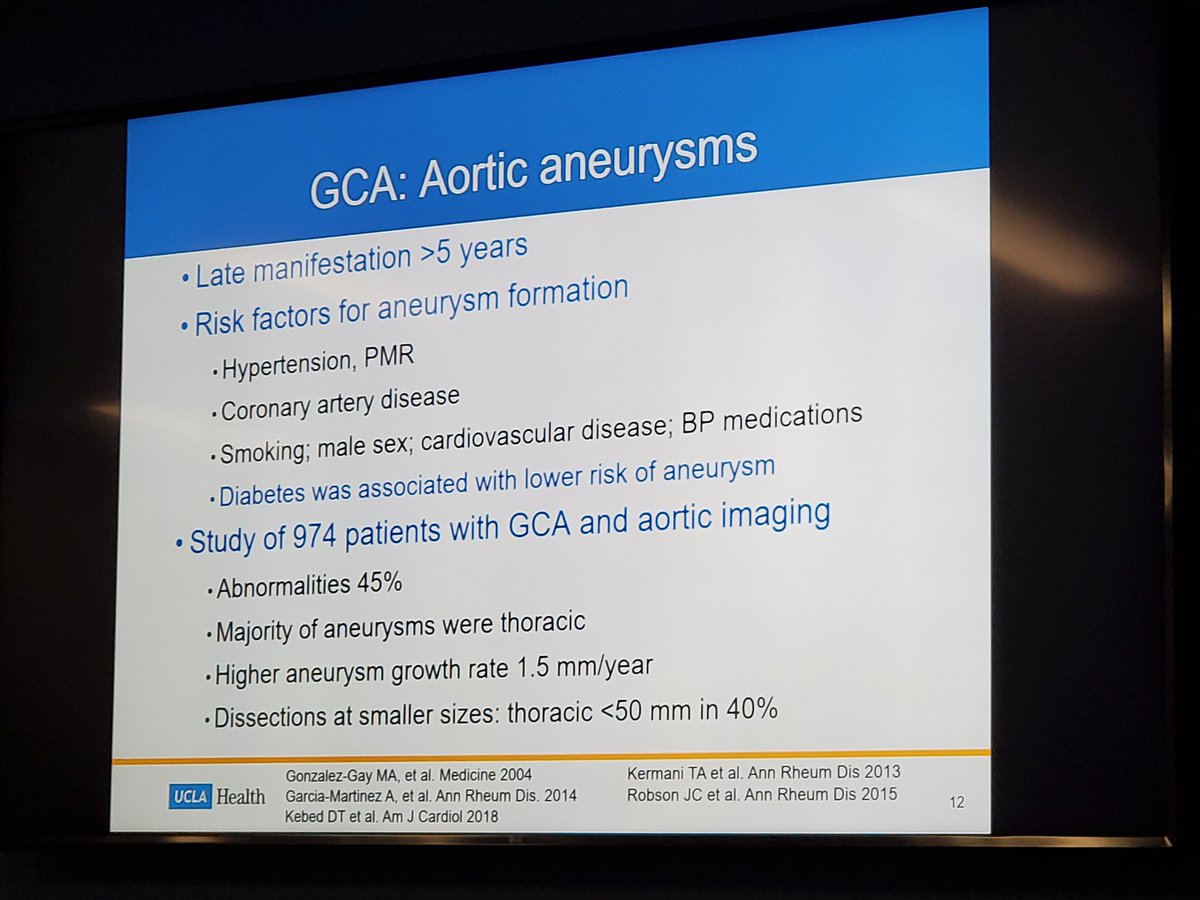

Dr. John Cush RheumNow ( View Tweet)



Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Prospectively study of neuropsychiatric (NP) events in1827 SLE - found that NP-SLE occured in 52%; with 31% directly attributed to SLE. Risk of NP-SLE was highest in the first 2 years (RR 6.16) and mortality risk was higher in NP-SLE pts ((16% vs 7%) https://t.co/03wfX5SxNm
Links:
Dr. John Cush RheumNow ( View Tweet)