ANA Pollution (2.06.2026)
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read Article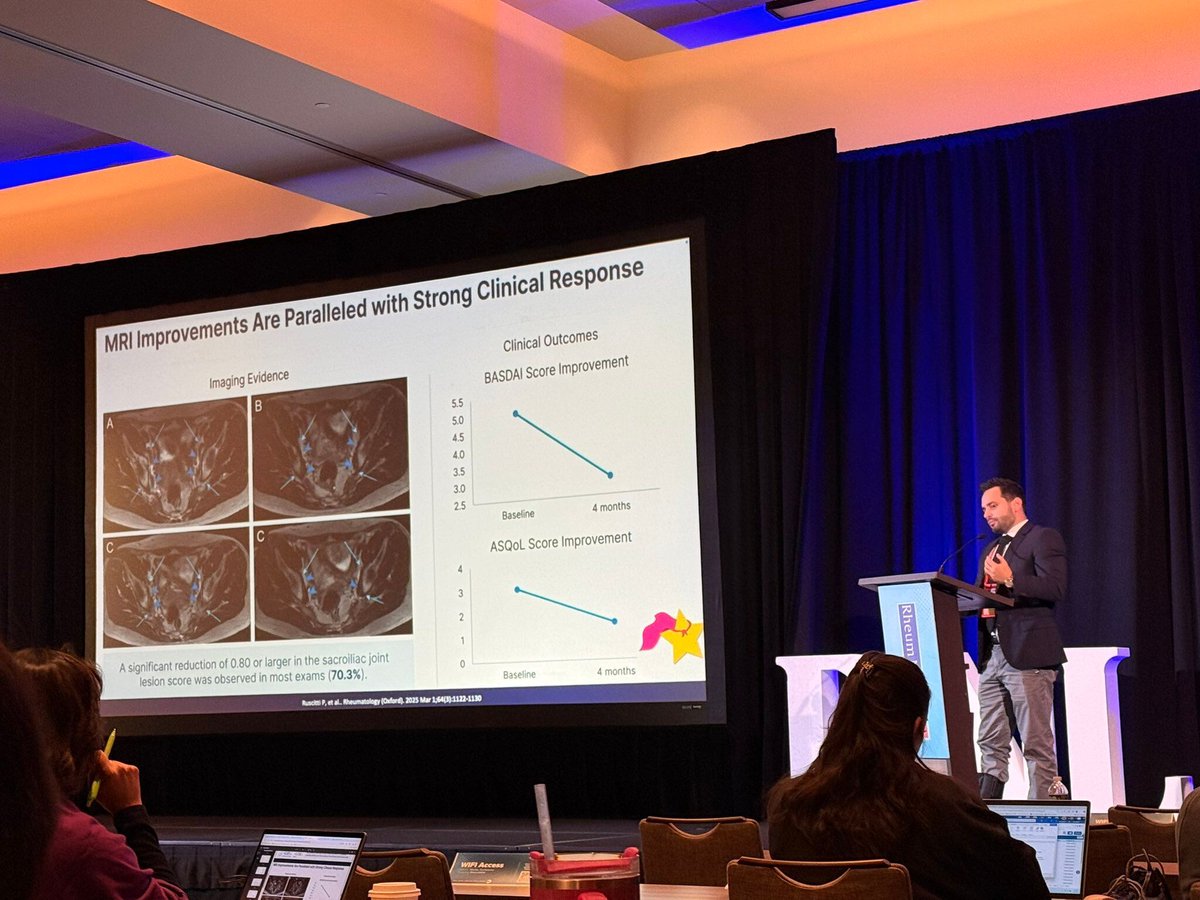
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read ArticleThe year 2025 presented numerous advances in rheumatology and related inflammatory and autoimmune disorders ranging from several new groundbreaking FDA approvals/indications, drug developments, game-changing guidelines and practices that will impact patient care for rheumatic diseases.
Read ArticleAdvanced practice clinicians (APCs; nurse practitioners and physician assistants) deliver a large share of US dermatologic care, accounting for 37% of clinicians and 27% of dermatology visits by 2020. A current JAMA Dermatology reports APC drug spending trends in dermatology, with a focus on
Read Article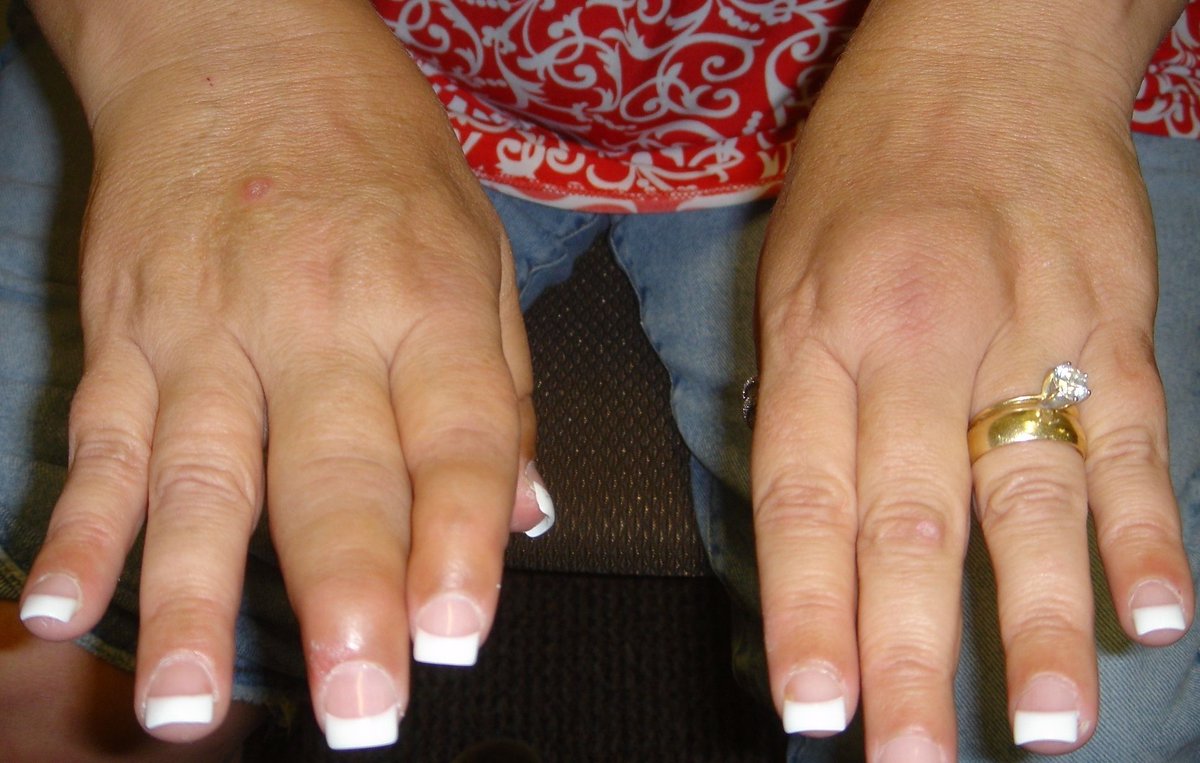
Guselkumab, a human IL-23 inhibitor, was evaluated in patients with active psoriatic arthritis (PsA) and shown to have both clinical and radiographic benefits at weeks 24 and 48.
Read Article

Drs. Jack Cush & Arthur Kavanaugh, two of rheumatology’s most trusted voices, provide a breakdown of the latest breakthroughs and hottest topics in rheumatology from the 2025 ACR Convergence meeting in Chicago.
Read ArticleHere's the last installment of our "ACR Best" abstracts as chosen by the RheumNow faculty. Most of these were from the final, day 4, but a few were noteworthy holdovers from day 3. Enjoy!
Read ArticleA new player is entering the IL-23 arena — and it’s a tablet! Icotrokinra (ICO), a first-in-class peptide that binds and blocks the interleukin-23 receptor (IL-23R), is showing encouraging efficacy and safety across a range of psoriasis (PSO) and psoriatic disease (PsD) studies.
Read Article
Links:
Nouf Al hemmadi @NoufAhmedAlham2( View Tweet )


Links:
Links:
Dr. Jack Cush reviews the news, FDA approvals, and journal articles. In this episode: HMGCR Abs, FDA approvals and Cush eats crow.
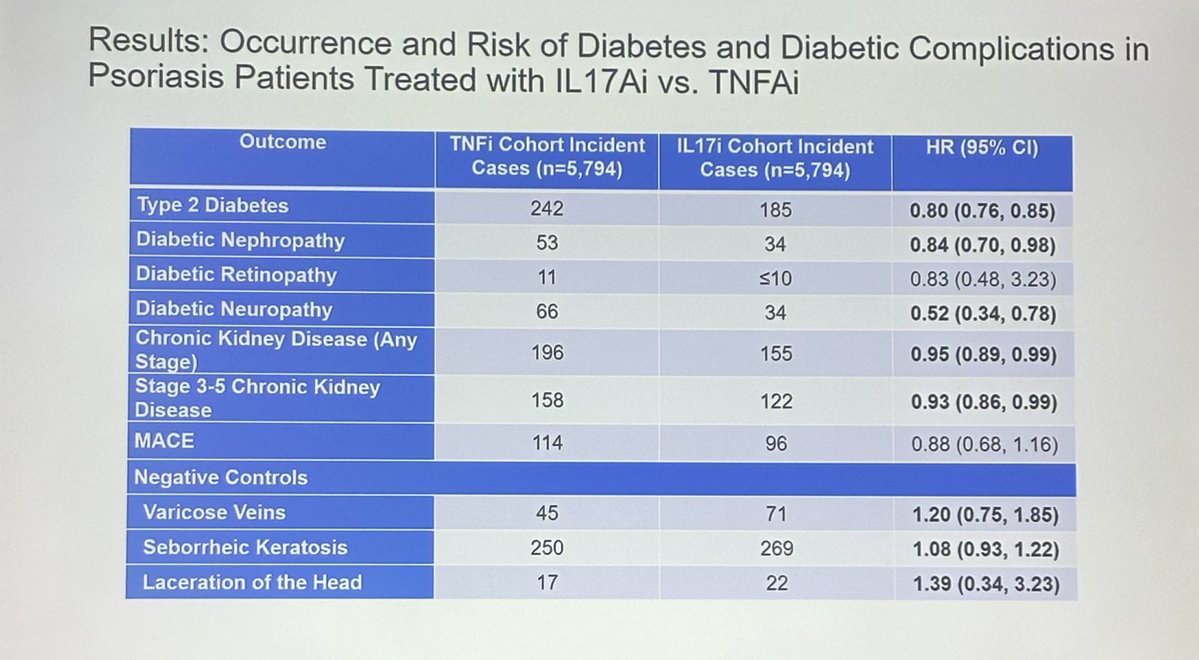
Read ArticleFDA announced yesterday that guselkumab (Tremfya) is approved for use in pediatric patients moderate to severe plaque psoriasis (PsO) or active psoriatic arthritis (PsA) in children six years and older (weighing at least 40 kg).
This is the first IL-23 inhibitor approved for use
Read Article
Dr. John Cush @RheumNow( View Tweet )
Treating psoriatic arthritis (PsA) patients with more than one targeted therapy appears to be just fine, with no increased risk of serious infections, insurance claims data indicate.
Read ArticleA systematic review and meta-analysis found that biologic switching was effective in a significant number of psoriasis patients; but there may be a price to pay.
Patients with psoriasis may be required to switch therapies, for a variet of reasons (
Although most patients with axial spondyloarthritis (axSpA) or psoriatic arthritis (PsA) didn't respond adequately to the interleukin-17 (IL-17) inhibitor ixekizumab (Taltz), enough did that it should still be considered in such cases, a Danish registry study indicated.
Read Article


Patients with autoimmune skin diseases (ASDs) with cancer had significantly better cancer survival outcomes than those without ASD, suggesting coexistant autoimmune or inflammatory disease does not adversely affect a cancer prognosis.
Read ArticleBy downloading this material, I acknowledge that it may be used only for personal use and personal education and that I will accredit RheumNow.com as the source and owner of this material. Commercial use or mass reproduction of this material without permission from RheumNow (info@rheumnow.com) is prohibited.