UBER Rheumatology Ride (3.6.2026)
Dr. Jack Cush reviews the news and journal reports from this week on RheumNow.com
Read Article
Dr. Jack Cush reviews the news and journal reports from this week on RheumNow.com
Read Article
As rheumatologists, most of us manage osteoporosis in some form every single day. We have newer agents, newer sequencing strategies, and an increasing number of patients asking for “less medicinal” options that go beyond a prescription. At RWCS, Dr. Gina Woods opened her talk by reframing
Read Article

Chimeric antigen receptor T (CAR-T) cell therapy has emerged as a transformative approach in modern medicine, demonstrating remarkable efficacy in targeting pathogenic B-cell lineages with unprecedented specificity. Originally developed for B cell malignancies, this innovative immunotherapy
Read ArticleI recently attended a fascinating lecture by Dr. Anisha Dua on progress in the diagnosis and treatment of large-vessel vasculitis, with a focus on giant cell arteritis (GCA) and Takayasu's arteritis. She opened with what felt like the most honest “state of the union” slide you can show a room
Read Article
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow.com.
Read Article

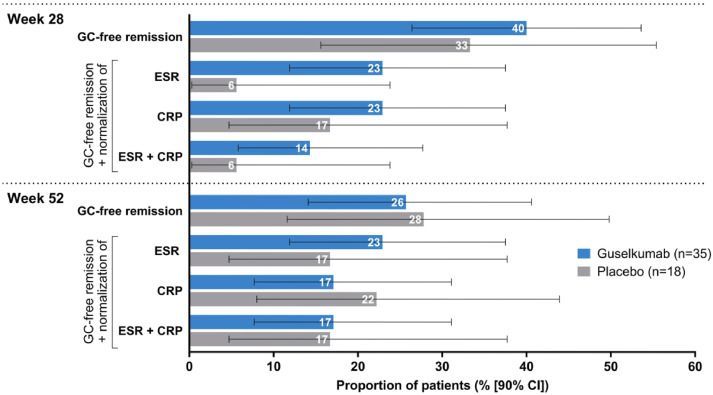
An economic evaluation of early vs delayed use of the Blys inhibitor, belimumab (BEL), in systemic lupus erythematosus (SLE) has shown both cost effectiveness and clinical utility of early BEL initiation in active lupus patients.
While most would advocate for using your best therapy
Read Article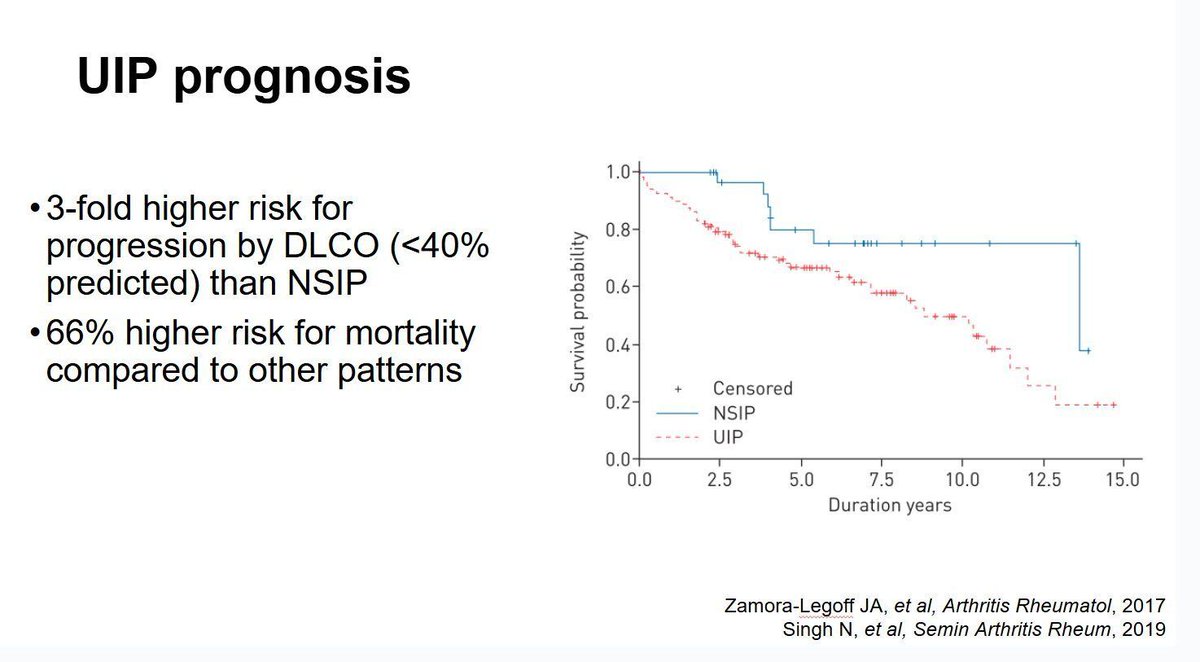
Giant-cell arteritis and Takayasu’s arteritis are both large-vessel vasculitides, but each brings its own diagnostic and management challenges. Many of the difficulties overlap because they are rare, heterogeneous, and lack perfect diagnostic tests or specific biomarkers.
Read Article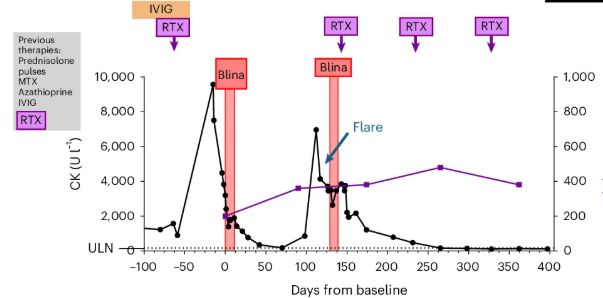
Currently, fewer than half of patients diagnosed with osteoporosis receive appropriate pharmacologic therapy. This treatment gap reflects several challenges in osteoporosis management, including limited disease awareness, barriers to medication access, clinician discomfort with therapy selection
Read ArticleToday Lilly announced top line results of the TOGETHER-PsO open-label, Phase 3b trial demonstrating the significant benefits of concomitant ixekizumab (IXE: an IL-17A inhibitor) and tirzepatide (TIR: GLP-1agonist) over IXE alone.
Read Article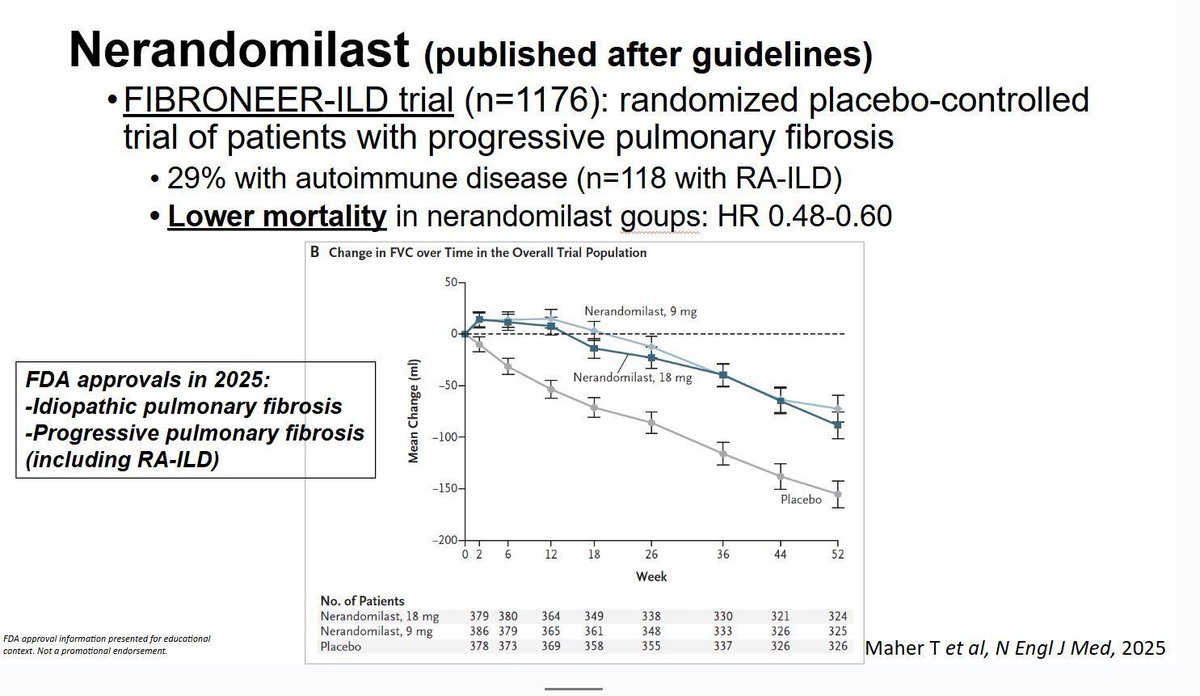
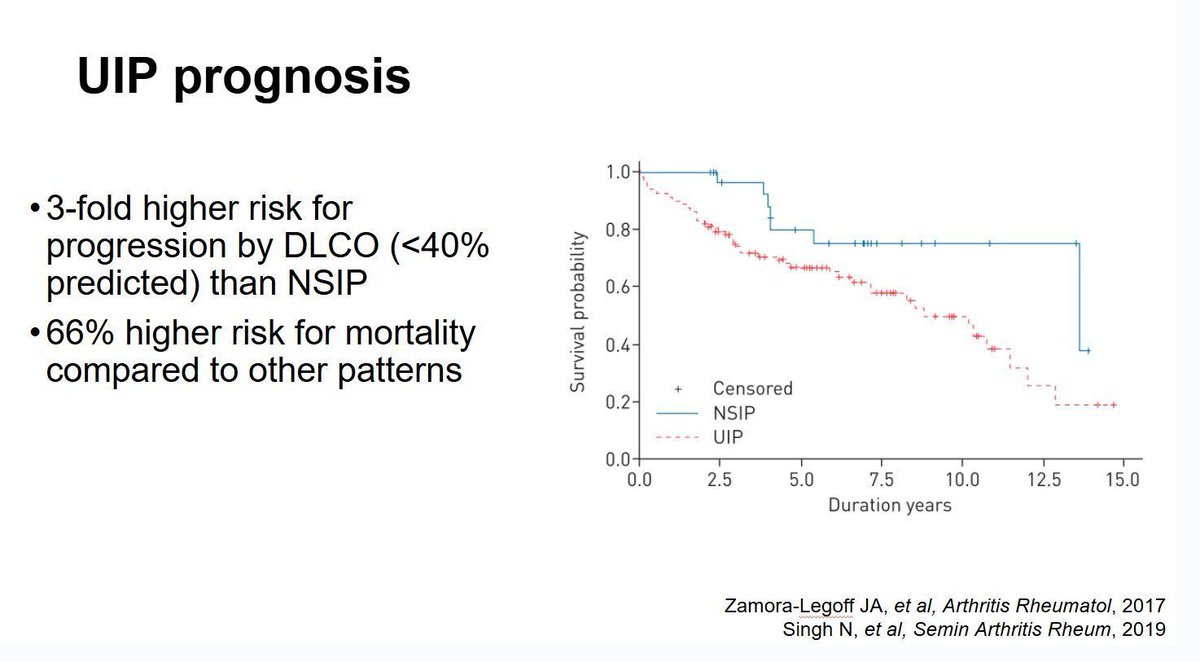
Gout management has entered what Dr. Robert Terkeltaub MD from UC San Diego described as its “disease-modifying era,” during his talk at RheumNow Live 2026. In a recent comprehensive review of the past, present, and future of gout therapy, the central message was clear: “We can really apply
Read ArticleRheumatologic care involves multidisciplinary approaches and collaboration with specialties to treat complex, systemic diseases. While many Pods at RheumNow Live are disease specific, the Pod II focused on Advancing Practice on important and emerging areas affecting rheumatologic patients. This
Read Article


By downloading this material, I acknowledge that it may be used only for personal use and personal education and that I will accredit RheumNow.com as the source and owner of this material. Commercial use or mass reproduction of this material without permission from RheumNow (info@rheumnow.com) is prohibited.