All News
EGPA in 2025
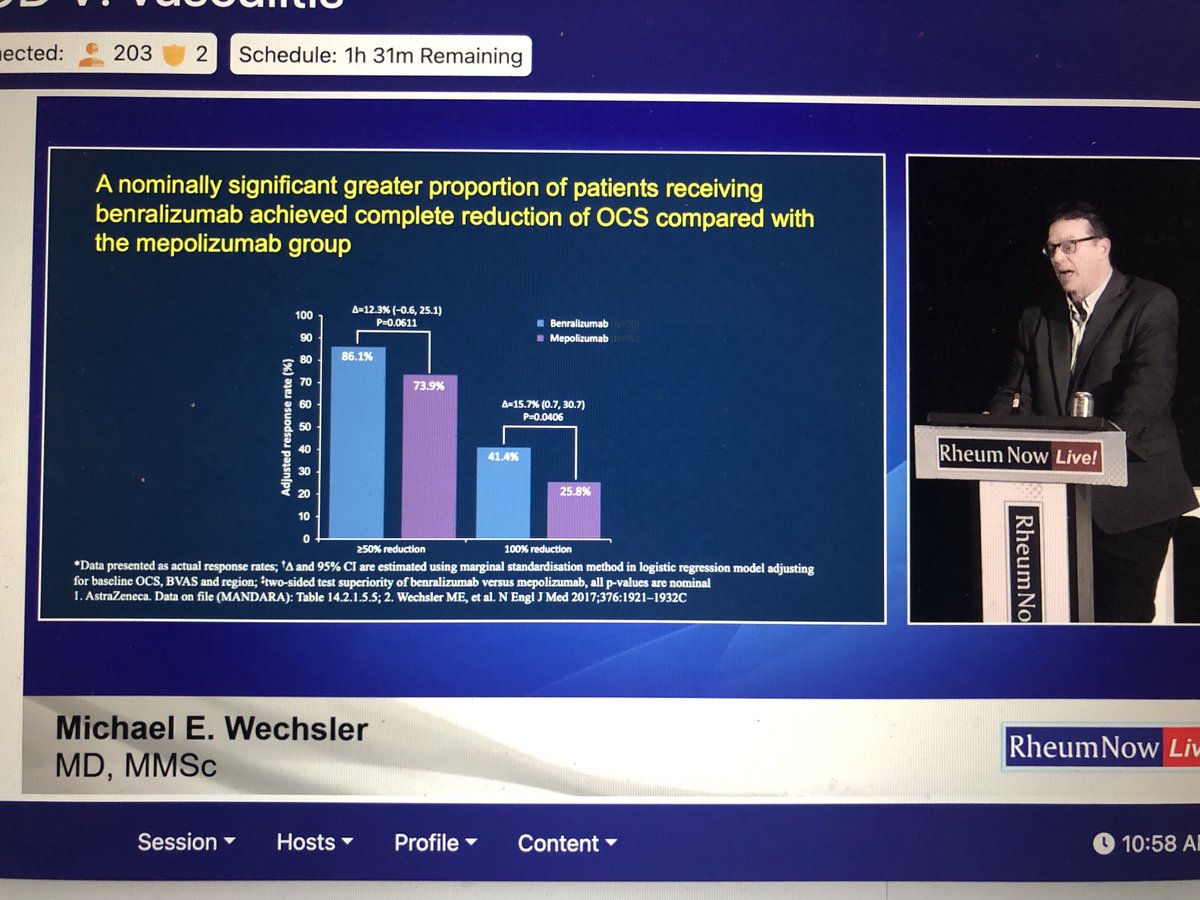
Formerly classified as an ANCA-associated vasculitis, EGPA is both most commonly ANCA negative and clinically different to the other two ANCA-associated vasculitis conditions, GPA and MPA. The management of EGPA has frequently fallen into the trap of being copied from its more common and well-known cousins. Now, however, we are seeing a discordance and following GPA/MPA management will potentially lead to both over-treatment and suboptimal treatment for EGPA. In this context, the Sunday morning session at RNL 2025 on “EGPA management in 2025 and beyond” by Dr. Michael Wechsler was both timely and clinically relevant.
Read ArticleThat's not my problem - or is it? Multimorbidity in RA
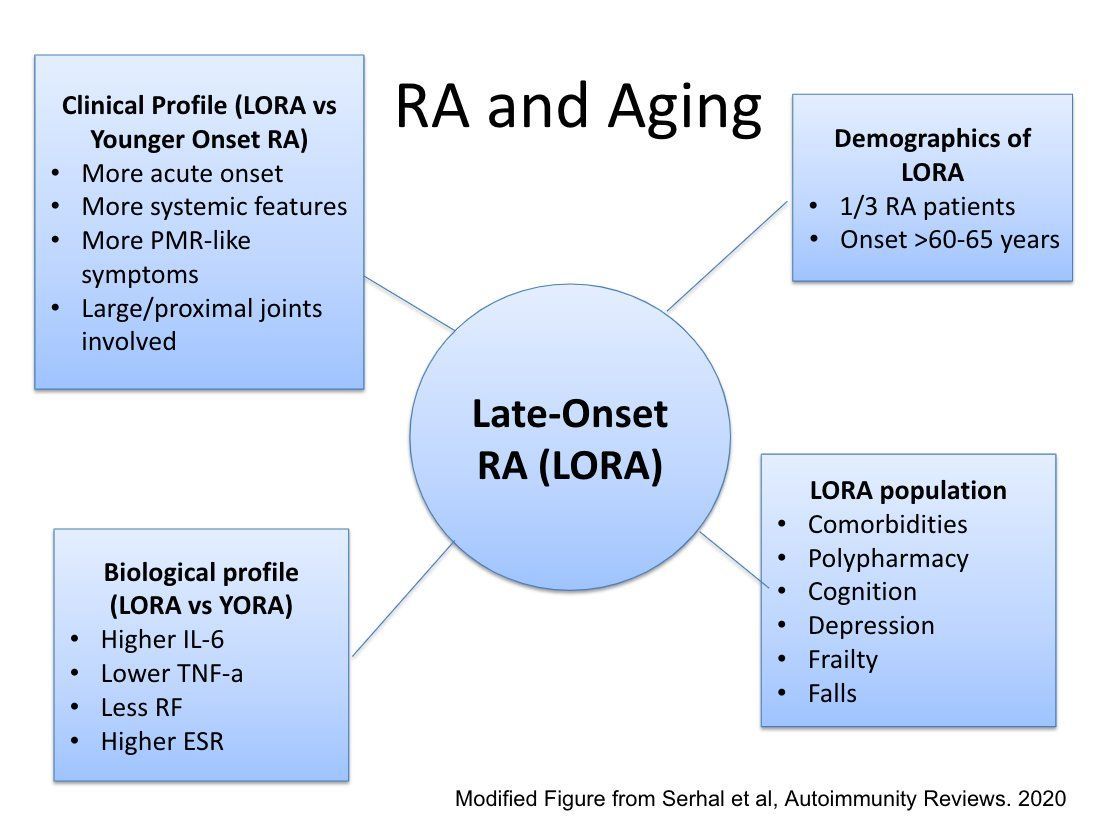
Traditionally, rheumatologists have often been deferential to the non-articular concerns in RA to other providers, though these issues are often left unaddressed. What role do rheumatologists have in the management of multimorbidity in our patients?
Read Article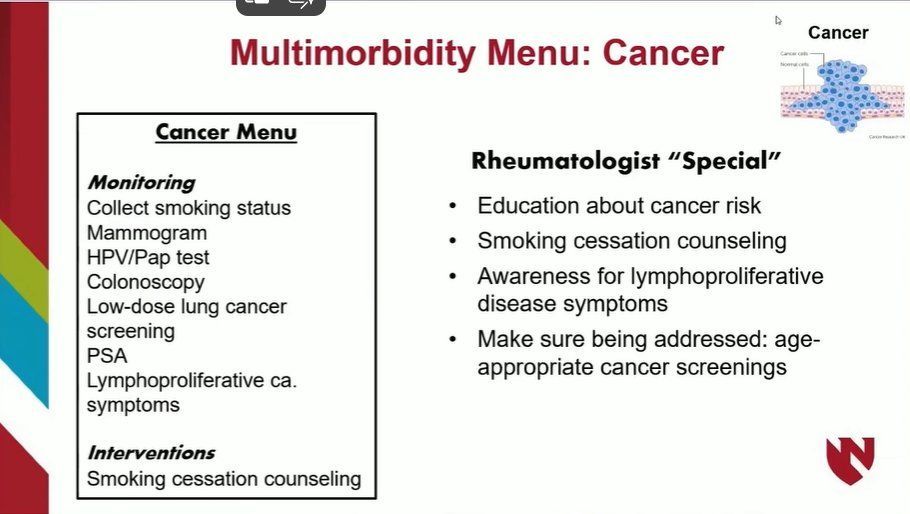
Links:

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Links:



Links: