All News

#RNL2025 @RheumNow
JAKi in DM:
@JuliePaikMD- 10 DM pts open label on JAKi. 7/10 improved from mod/severe to mild. In 96wk extension, 6 of 7 continued response
145 case reports for tofa, bari, ruxolitinib.
Role in helping calcinosis? Pruritu?
Brepocitinib (JAK1/TYK2i) study
Eric Dein ericdeinmd ( View Tweet)
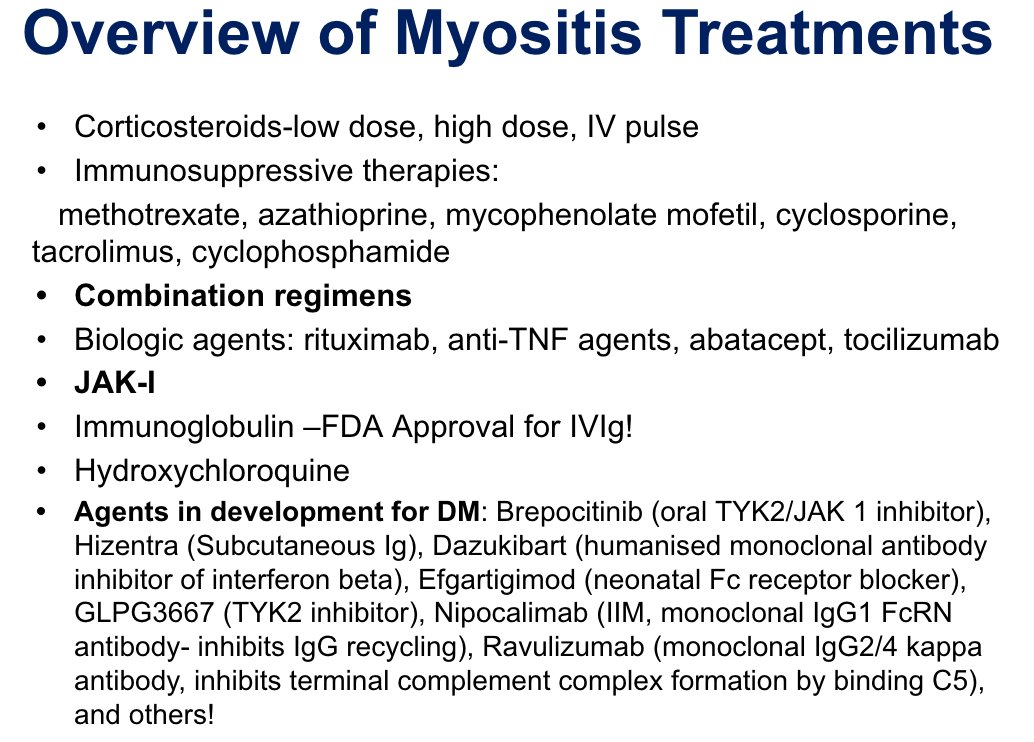
Pearls on Dermatomyositis:
-Negative myomarker does not r/o DM
-Pruritus can be a skin manifestation in DM🚨
-Combination therapy upfront =achieve faster remission
-Evidence for JAK inhibitors in management
-JAKis seem to have good effect in DM-calcinosis
-In DM with rapidly… https://t.co/J3iiDa6ph7 https://t.co/0WTHKa97bt
Links:
Adela Castro AdelaCastro222 ( View Tweet)
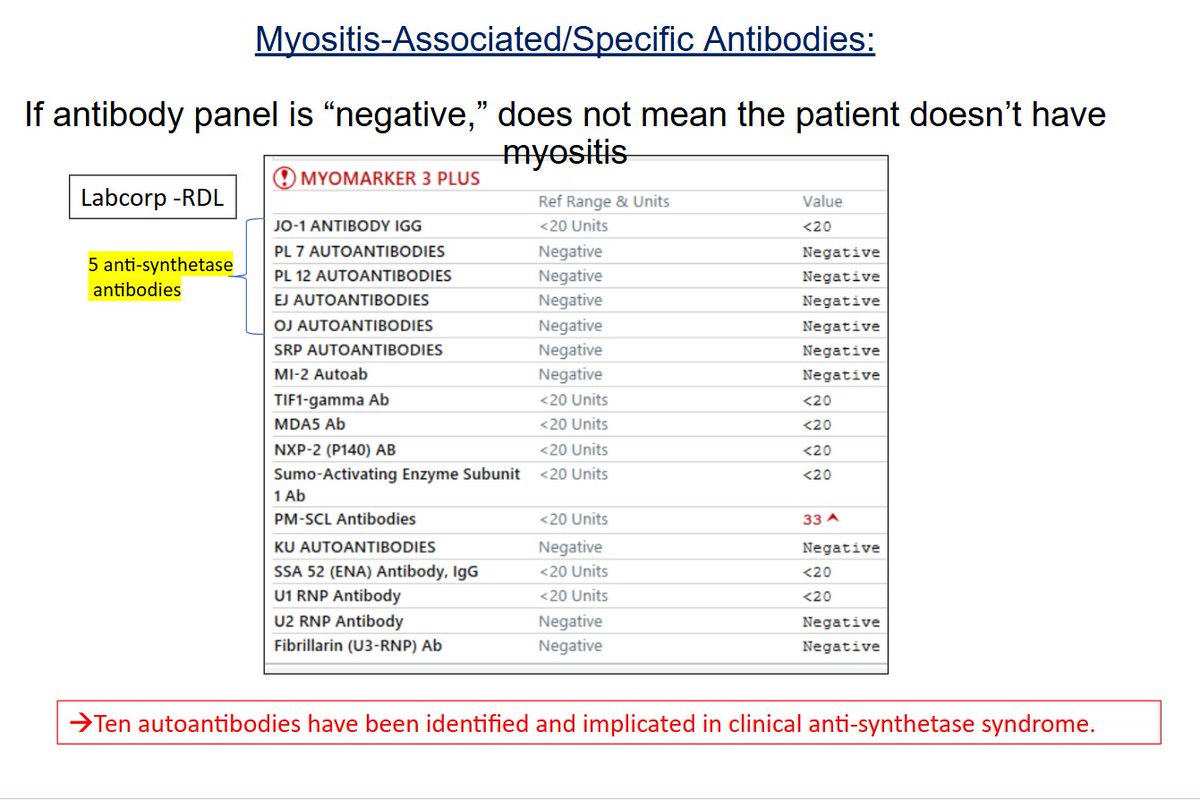
Importance of not over-relying on lab tests. We can test for what we can test for, but this is not comprehensive. For me in clinical practice - Postive antibodies can confirm diagnosis of DM but negative cannot exclude it. @RheumNow #RNL2025 https://t.co/Kyfzne4BSD
Richard Conway RichardPAConway ( View Tweet)

Do you want to know a secret? @drcharitydean shares that doctors are wired in the same way that #AI is built. We understand the layers of complexity & are best equipped to partner with AI. @rheumnow #RNL2025
TheDaoIndex KDAO2011 ( View Tweet)

"Treat for the most likely, mitigate for the catastrophic" @drcharitydean (my hero!). Read her story in "Premonition" by Michael Lewis. She served an impt role in the COVID19 pandemic as a public health officer for CA. Her lecture is on re-inventing yourself.
@rheumnow #RNL2025 https://t.co/3ZWgClzez1
TheDaoIndex KDAO2011 ( View Tweet)
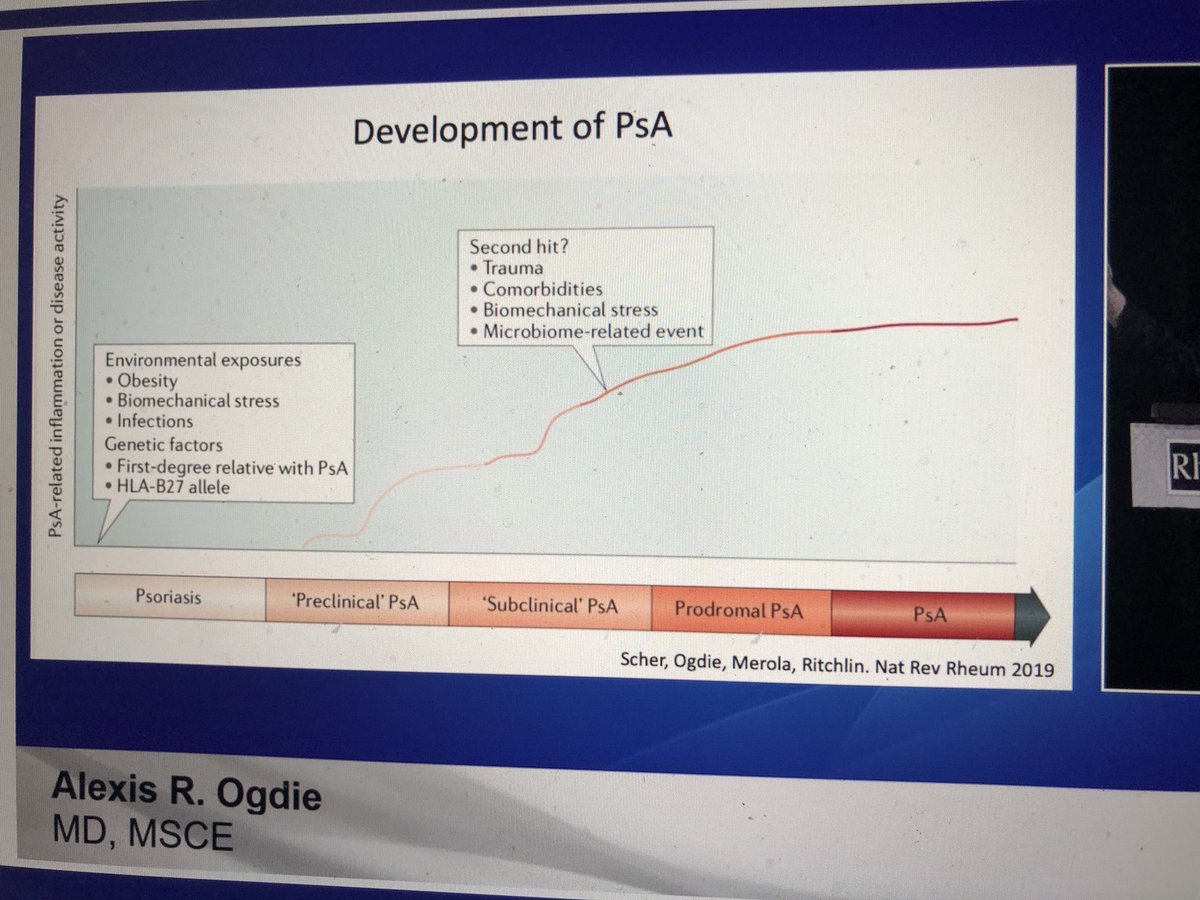
#HOT #topic
Will #Rx of #PsO PREVENT onset of #PsA
Well, maybe 🤔
Alexis R Ogdie @RheumNow #RNL2025
70% of #psoriatic #arthritis is preceded by #psoriasis
Still a bit of a debate re prevention of PsA https://t.co/7bce8kcjdr
Janet Pope Janetbirdope ( View Tweet)
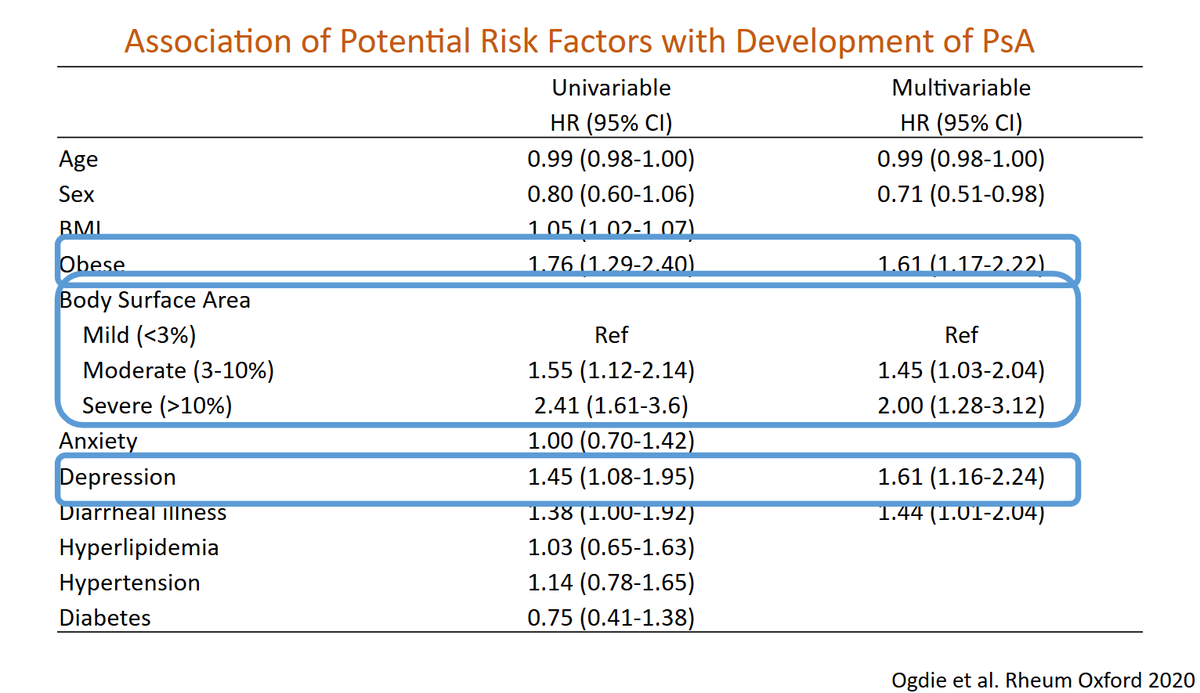
Risk factors for development of PsA in psoriasis. Psoriasis severity, obesity, and depression. @alexisogdie @RheumNow @RNL2025 https://t.co/obMDBvLLnx
Richard Conway RichardPAConway ( View Tweet)
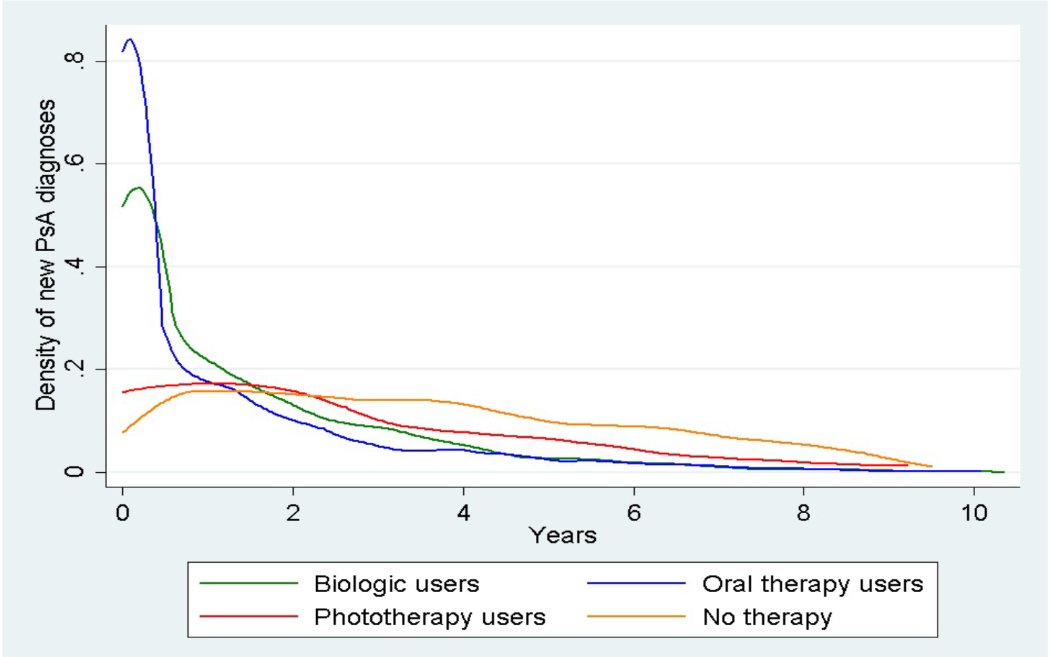
Great graph from @AlexisOgdie on the effect of biologic treatment on PsA development in psoriasis. Obvious front-loading in those who need systemic therapy. But over time those who don't appear to develop more PsA @RheumNow @RNL2025 https://t.co/UxUZyKb0NA
Richard Conway RichardPAConway ( View Tweet)
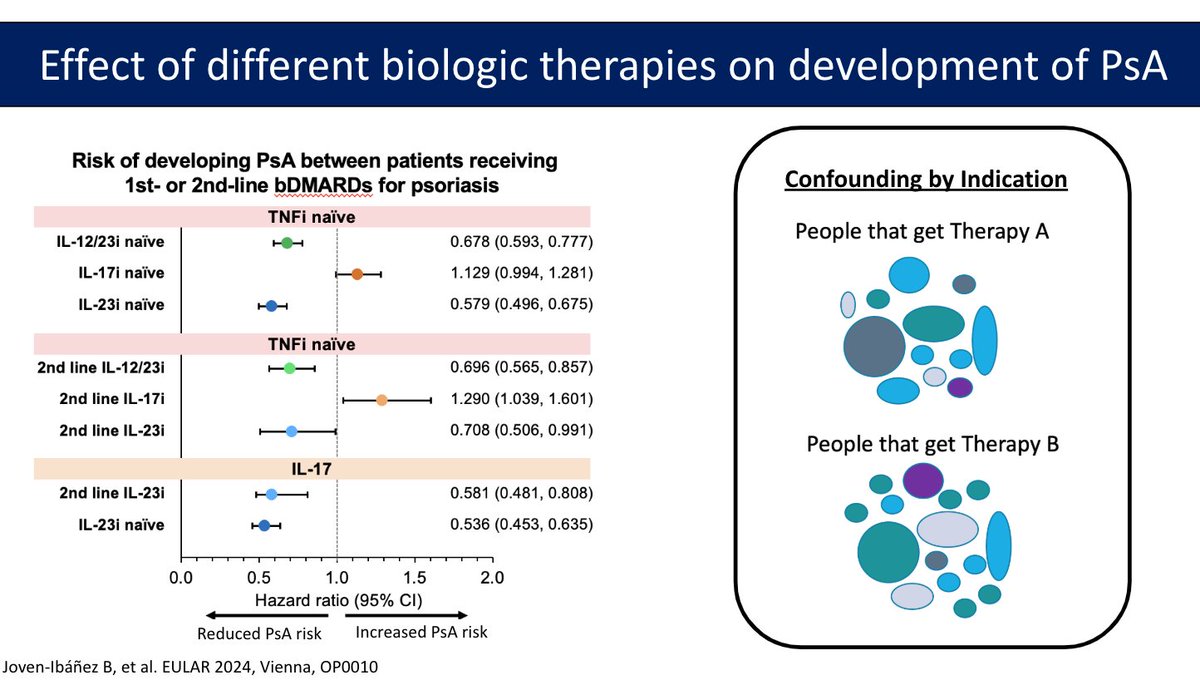
Can treatment of PsO prevent PsA?
-Severity of PsO and obesity->Risk fx for PsA
-PRESTO scores: Predicts whether you will develop PsA in the next year vs next 5 years
-Does ttx of PsO impact development of PsA?
Trials are mostly observational (several confounders)
TriNetX data:… https://t.co/TBv3Y2TLZv https://t.co/1K0MtMQ5gn
Links:
Adela Castro AdelaCastro222 ( View Tweet)

FDA has approved Journavx (suzetrigine) 50 mg tablets, 1st in-class non-opioid analgesic, to treat moderate to severe acute pain in adults. Journavx reduces pain by targeting a pain-signaling pathway involving sodium channels in the peripheral nervous system, before pain signals… https://t.co/qjtr4yz8Ot https://t.co/RUZmLgsmTv
Dr. John Cush RheumNow ( View Tweet)
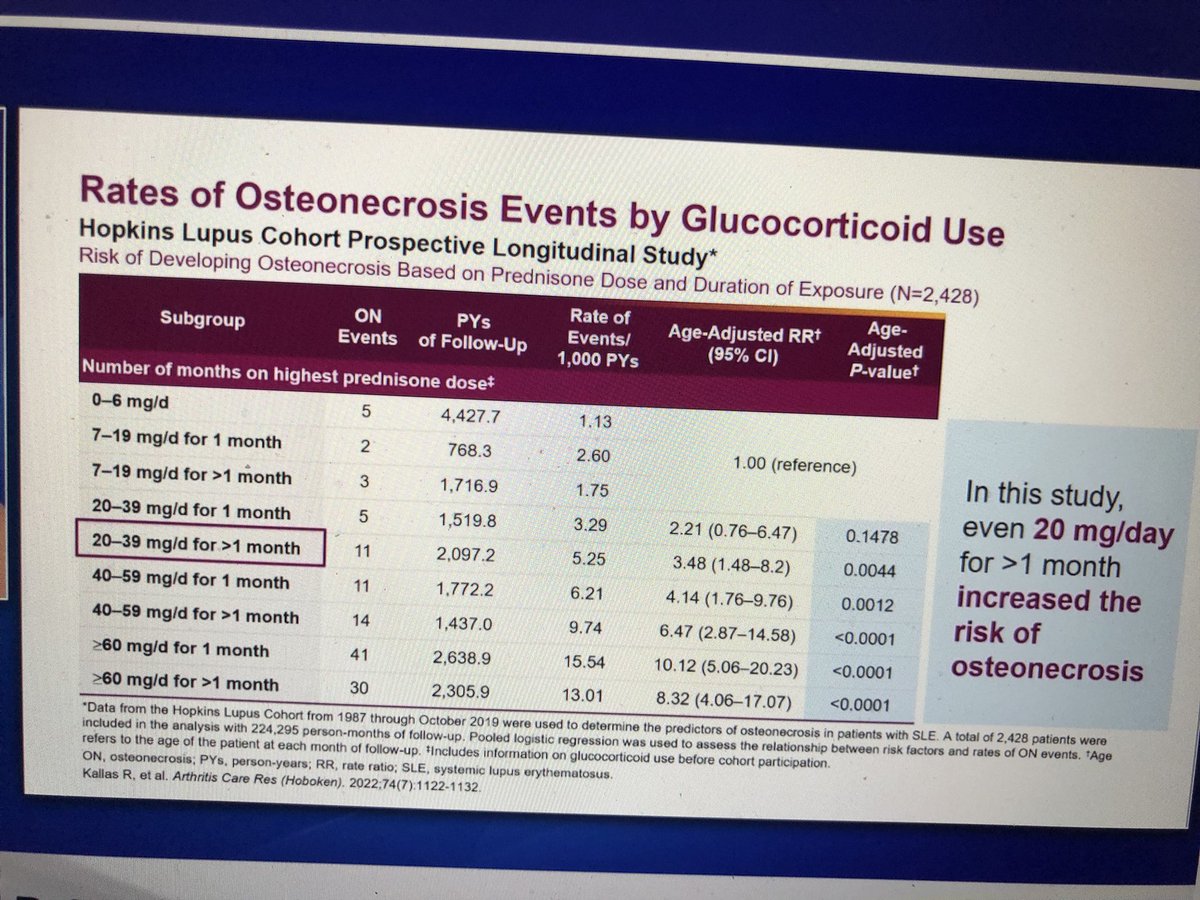
Break a leg! Or a #hip
#AVN risk occurs even at 1 month of high / moderate dose #glucocorticoids
Shown by Michelle Petri @RheumNow #RNL2025
Risk in SLE is likely
👇
#disease #activity
And
#steroids
#SLE #systemic #lupus #erythematosus #steroids https://t.co/4EsQw9tY1u
Janet Pope Janetbirdope ( View Tweet)
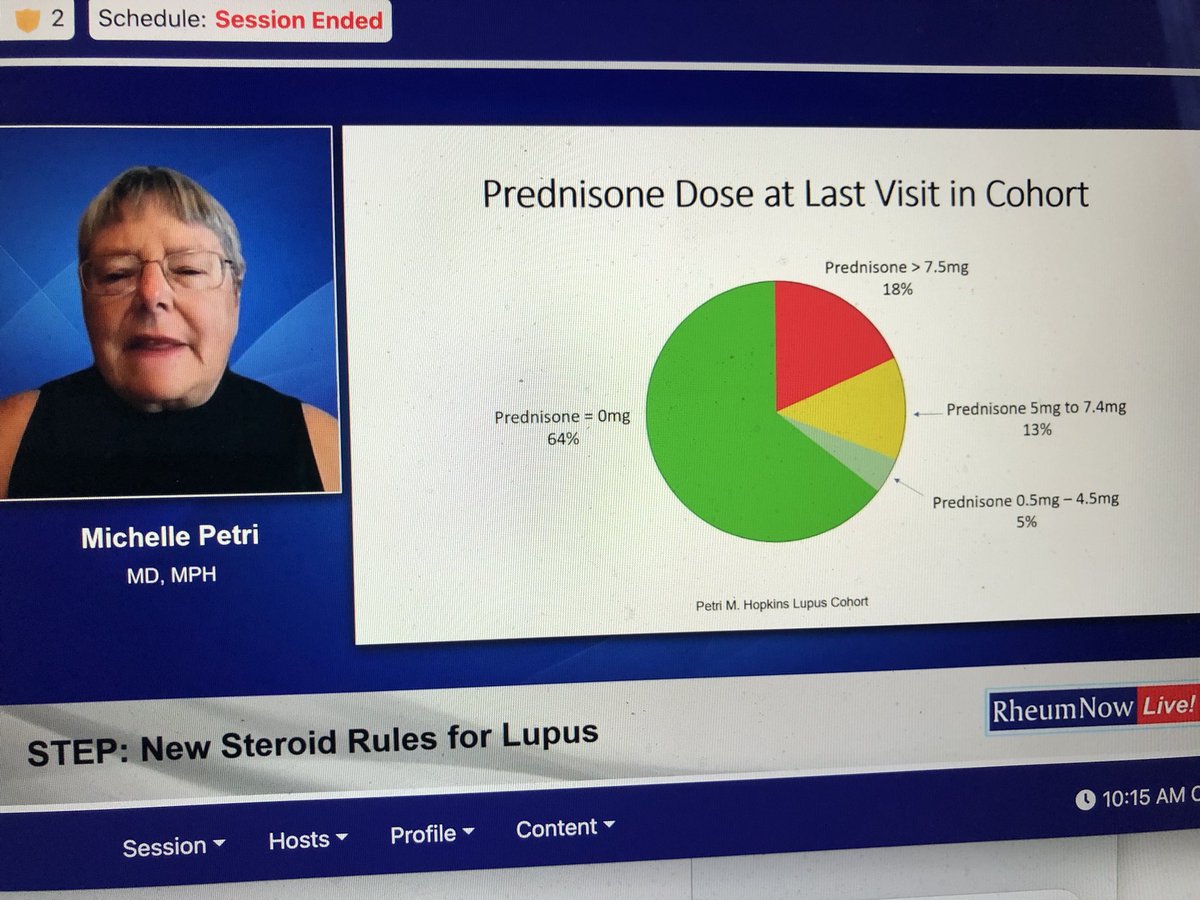
Even a #SLE expert who wants all #lupus Pts on
No #chronic #prednisone
Realizes you can’t get everyone off #glucocorticoids
18% May be on chronic #steroids
But she keeps trying to taper
Michelle Petri
#RNL2025 @RheumNow https://t.co/SNP6Eep731
Janet Pope Janetbirdope ( View Tweet)

M. Petri on Steroids in SLE👇
-Organ damage is higher in AA vs Caucasians-driven by CS
-CV risk increase is dose dependent:
>10 mg =2x risk
>20mg =5x risk
-Increase prednisone by 10mg =30% increased risk for organ damage! 🤯
-20mg/day >1m increases risk for… https://t.co/fZVjS7JQBL
Adela Castro AdelaCastro222 ( View Tweet)

QD Clinic - dsDNA without Lupus
Insufficient Sxs to Dx SLE, but persistent dsDNA positivity - what to do?
Features Dr. Jack Cush.
QD Clinics - lessons from the clinic, sponsored by RNL2025 in Dallas, TX; Feb 8 & 9, 2025
Register at https://t.co/2dcFVgu8z6… https://t.co/hCXRhTJB7G https://t.co/y3Tzh5bRLJ
Links:
Dr. John Cush RheumNow ( View Tweet)

Wise person once said
If you don’t take your medications,
THEY DON’T WORK!
Shocking 1 in 3 nonadherence with
#hydroxychloroquine
By drug levels
In young Pts with
#SLE #lupus
Michelle Petri @RheumNow #RNL2025 https://t.co/H5izbnW0qU
Janet Pope Janetbirdope ( View Tweet)

Michele Petri @RheumNow #RNL2025
1 mg of prednisone -> 3% increased risk of damage
Taper quickly, withdrawal slowly
Make sure background therapy is on - monitor therapy with HCQ levels, early use of additional Rx https://t.co/pjFNcg99ed
Eric Dein ericdeinmd ( View Tweet)
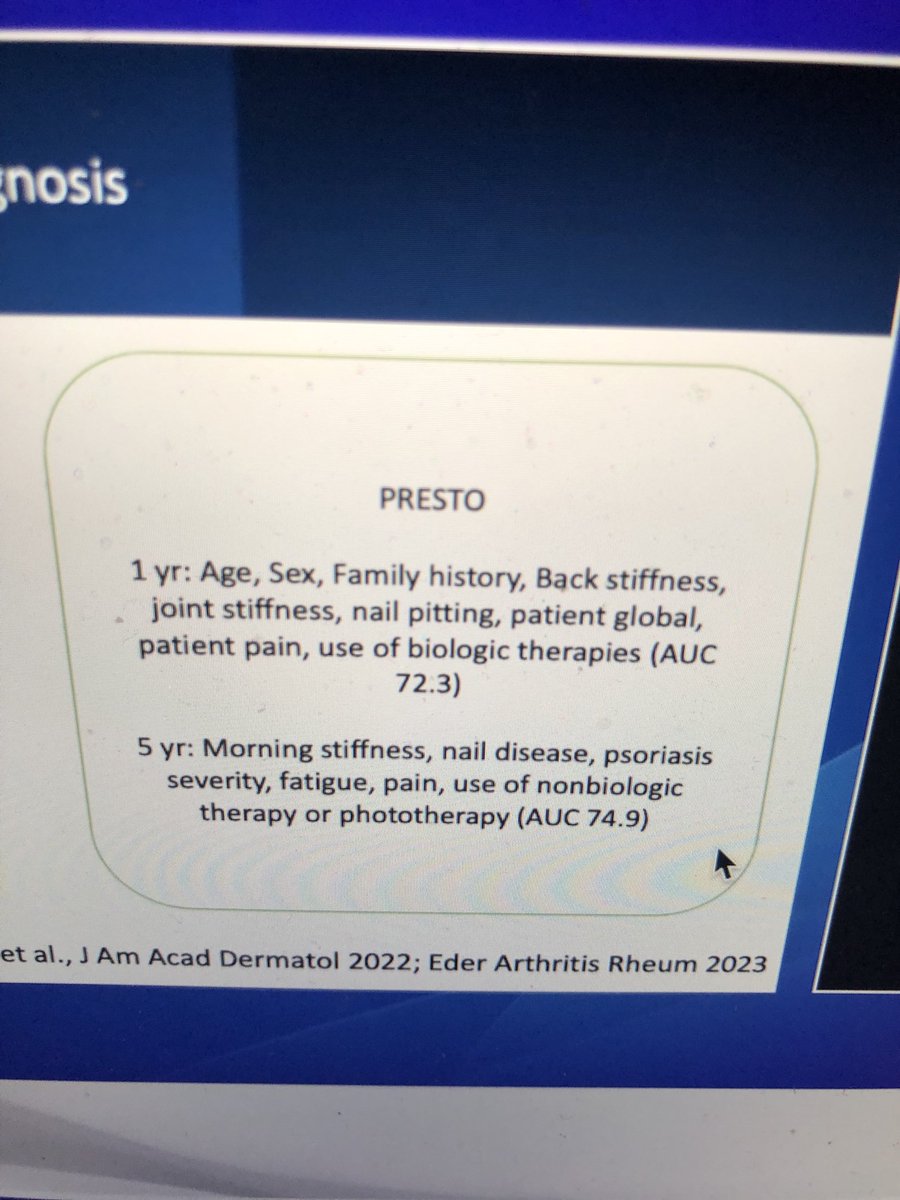
Predicting #PsA
PRESTO study #obvious risks are #predictive
Alexis R Ogdie @RheumNow #RNL2025
bad PsO 2X ^PsA
High BMI
Depression
Modifiable risks #psoriasis #psoriatic #arthritis https://t.co/pOxidCWcg5
Janet Pope Janetbirdope ( View Tweet)
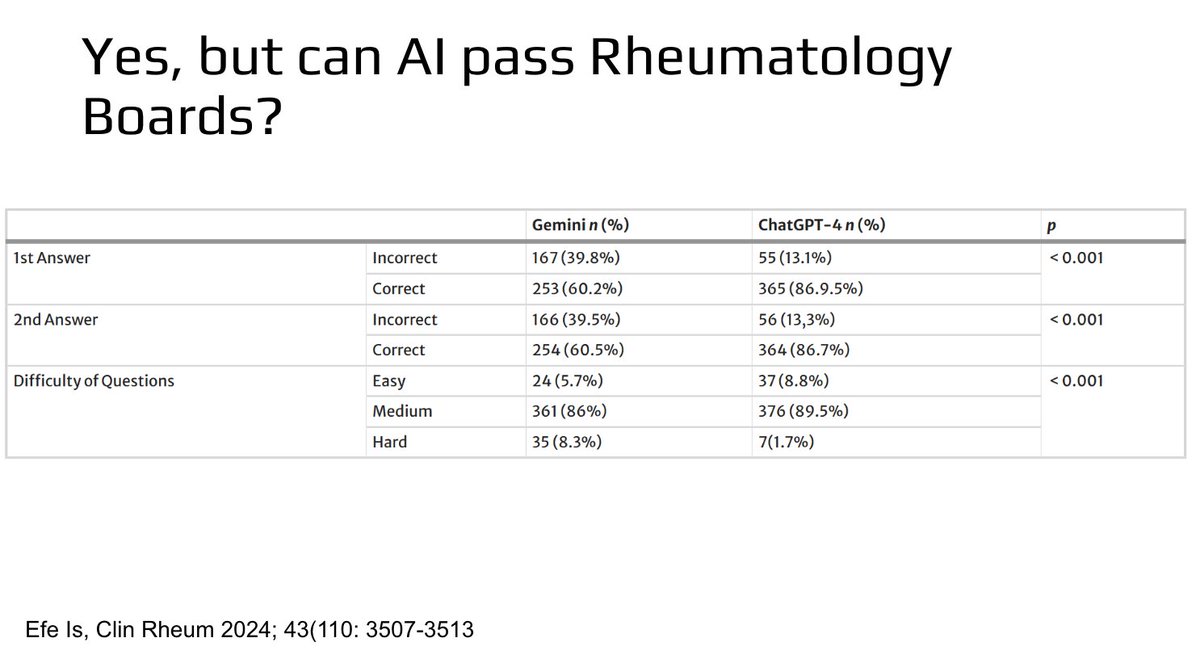
Can AI pass the rheumatology boards? Yes! 🤯
Stay tuned for more interesting rheumatology pearls at RheumNow Live 2025!
#RNL2025 @RheumNow https://t.co/bNurQlH5OU
Adela Castro AdelaCastro222 ( View Tweet)
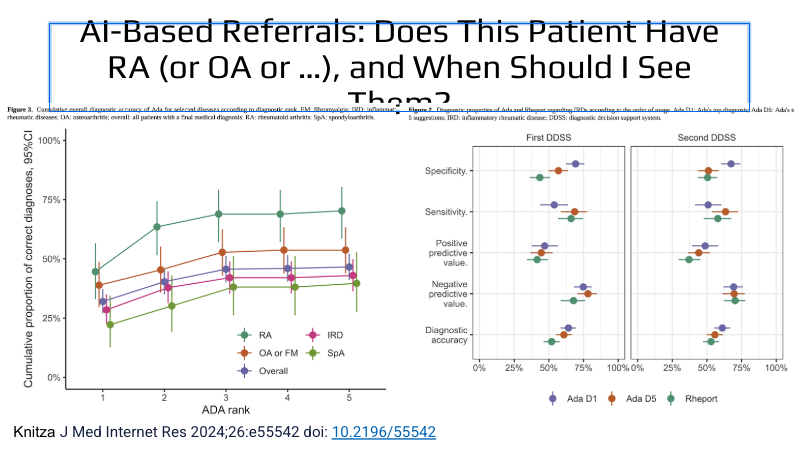
🏈Kick-off of @RheumNow w Jeff Curtis #RNL2025 🏈
AI in RA?
Can help with Rheum Boards Questions, though AI is not confident in answers
May not be better than paper charts for triage
Other roles: pt chatbot, AI scribe, research populations, generate DDx, consume guidelines https://t.co/X43DCmt0Ov
Links:
Eric Dein ericdeinmd ( View Tweet)

Who’s liable for an incorrect #AI diagnosis or medical advice? @rheumnow #RNL2025 @RADoctor
TheDaoIndex KDAO2011 ( View Tweet)



