All News
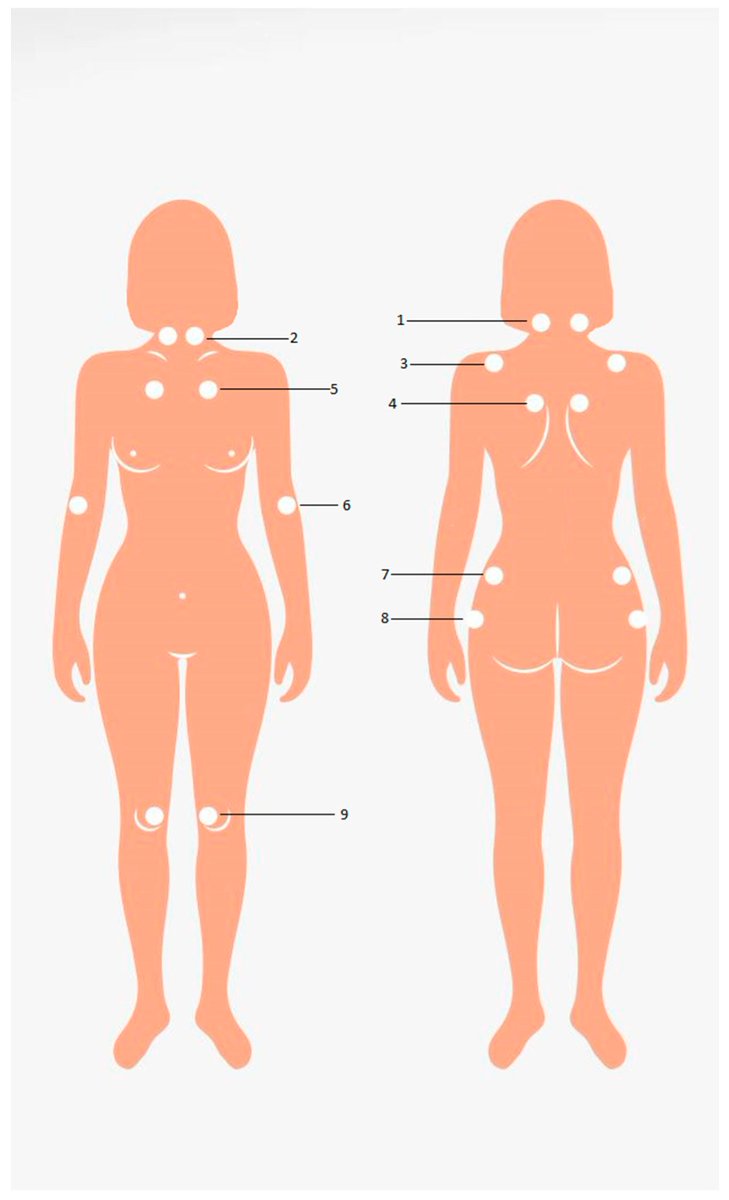
Pain Management in Rheumatoid Arthritis
BMC has published a full read literture review of pharmacological pain management in rheumatoid arthritis, pointing out that the best evidence for pain control comes from the use of DMARDs and treat-to-target strategies.
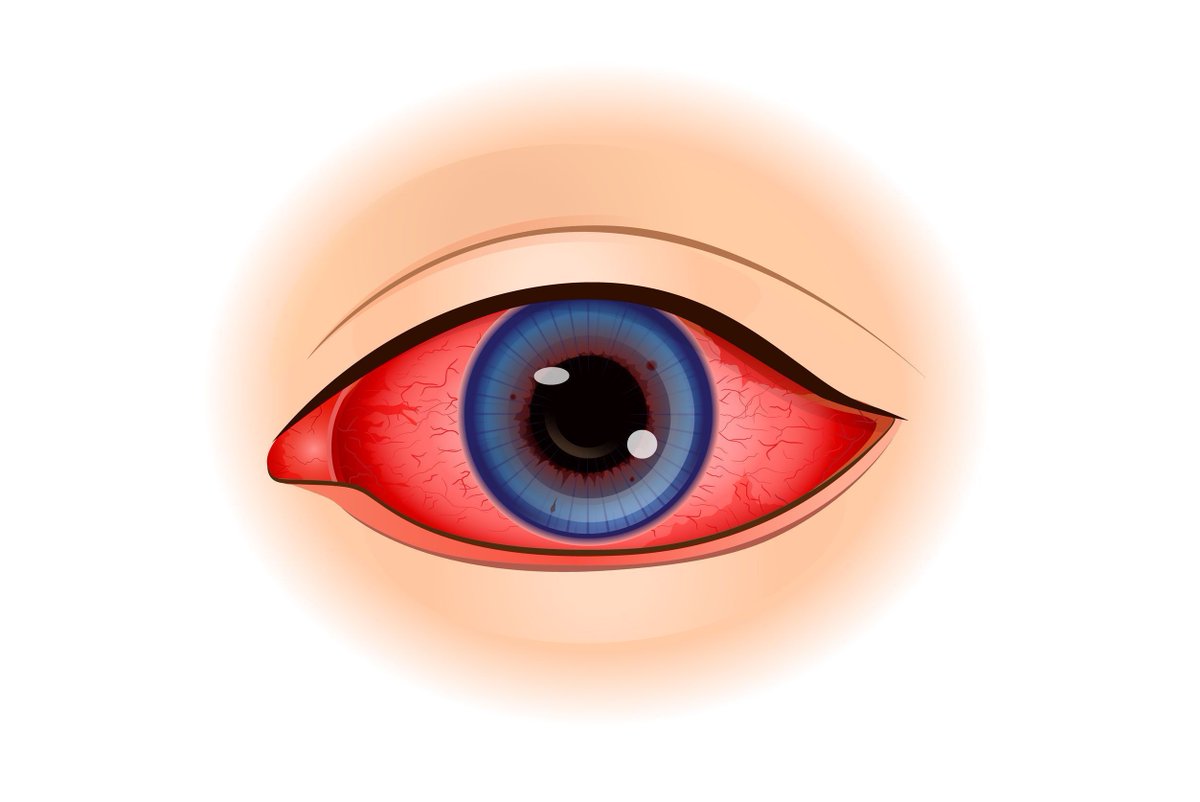
Read ArticleBSR Guideline on Management of Sjögren disease
The British Society for Rheumatology has published its guideline for the management of adult and juvenile onset Sjogren's disease.
Read ArticleRecent Excess Mortality in Young Adults
JAMA has published a University of Minnesota cross-sectional study showing early adult (ages 25-44) mortality in the U.S. has risen substantially between 2011 to 2019 and 2020 to 2023. The largest portion of 2023 excess mortality was driven by drug poisoning, but many other external and natural causes exceeded what prior trends would have projected.
Read ArticleBlood Flow Restriction to Reduce Pain in Knee OA
A technique now widely used in sports medicine to speed recovery from leg injuries helped reduce symptoms and improve function in people with osteoarthritis (OA) of the knee, a randomized trial showed.
Read Article