All News
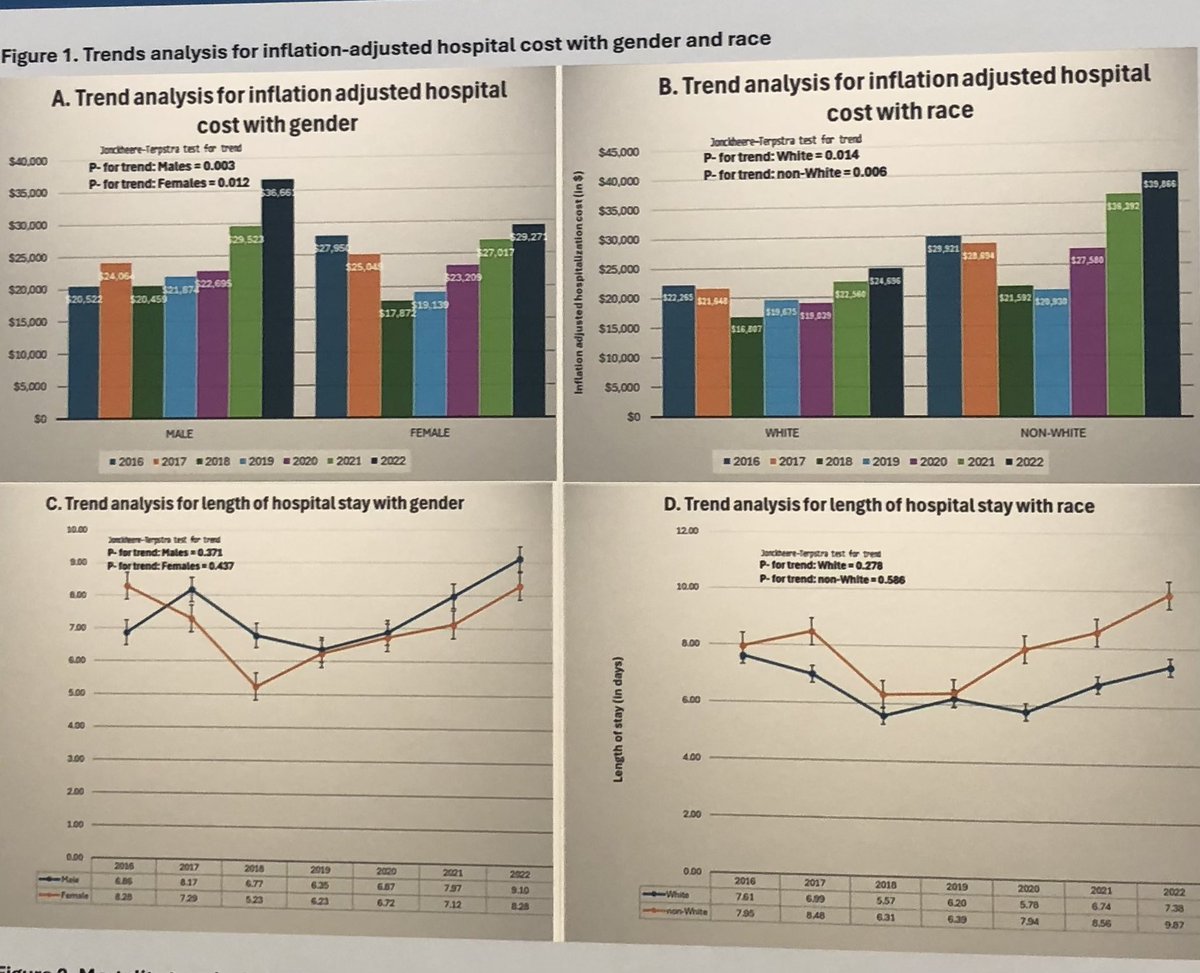
Why do pts with #Still’s #AOSD
Get hospitalized?
#Sepsis
1827 unweighted & 9135 Weighted #US #hospitalizations
Worse in
men
Black
Medicaid
But is it truly #infection or Still’s flare or both?
Abst#0163
ACR25 @RheumNow @rheumacr
#ACRBest as largest N ever! https://t.co/fLMNyQQNNj
Janet Pope Janetbirdope ( View Tweet)
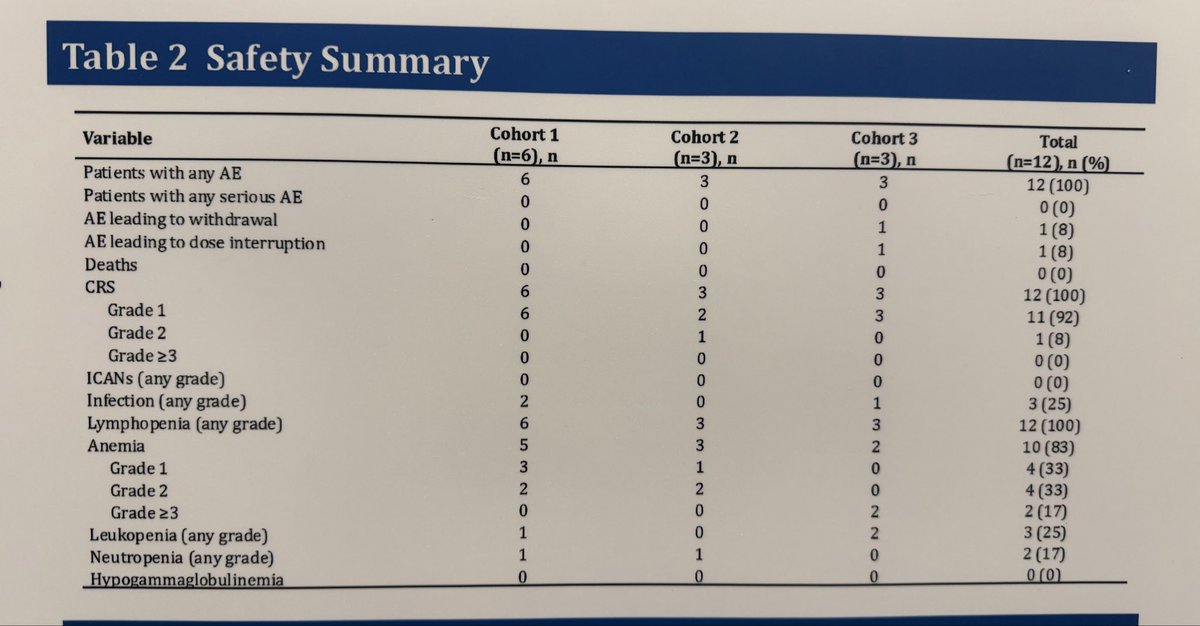
#ACR25 Beyond blinatumomab, A-319, CD3xCD19 BiTE in 12 patients with #SLE in China reported dose-dependent depth of B-Cell depletion but not efficacy or safety. One withdrew due to AE. No CRS/ICANS. Be interested to see results in larger trials & longer follow-up @RheumNow https://t.co/e5RuZlFI6i
Links:
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)
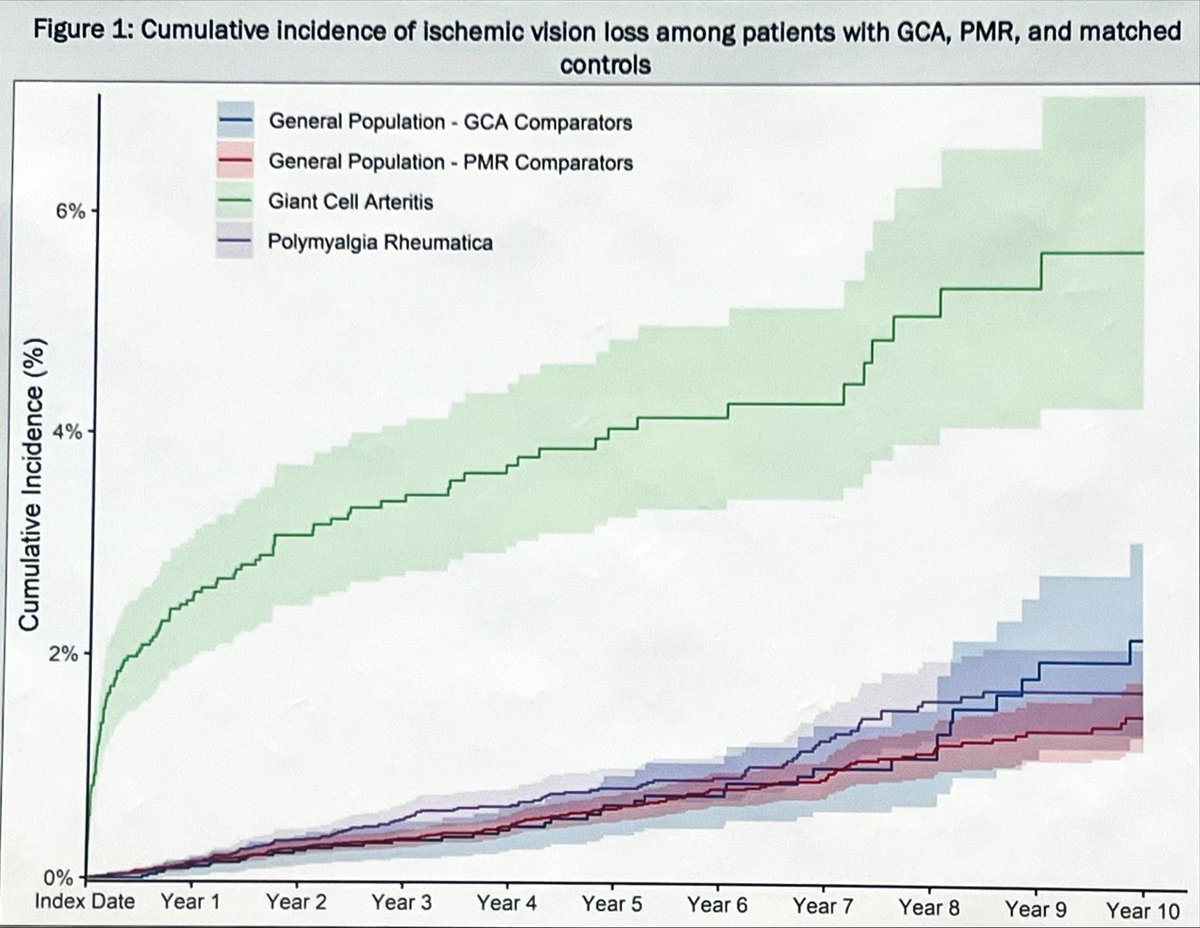
In case there was any doubt:
GCA leads to ischemic vision loss
PMR does not
#ACR25 ABST0753 @RheumNow https://t.co/mCS9B4Flgl
David Liew drdavidliew ( View Tweet)
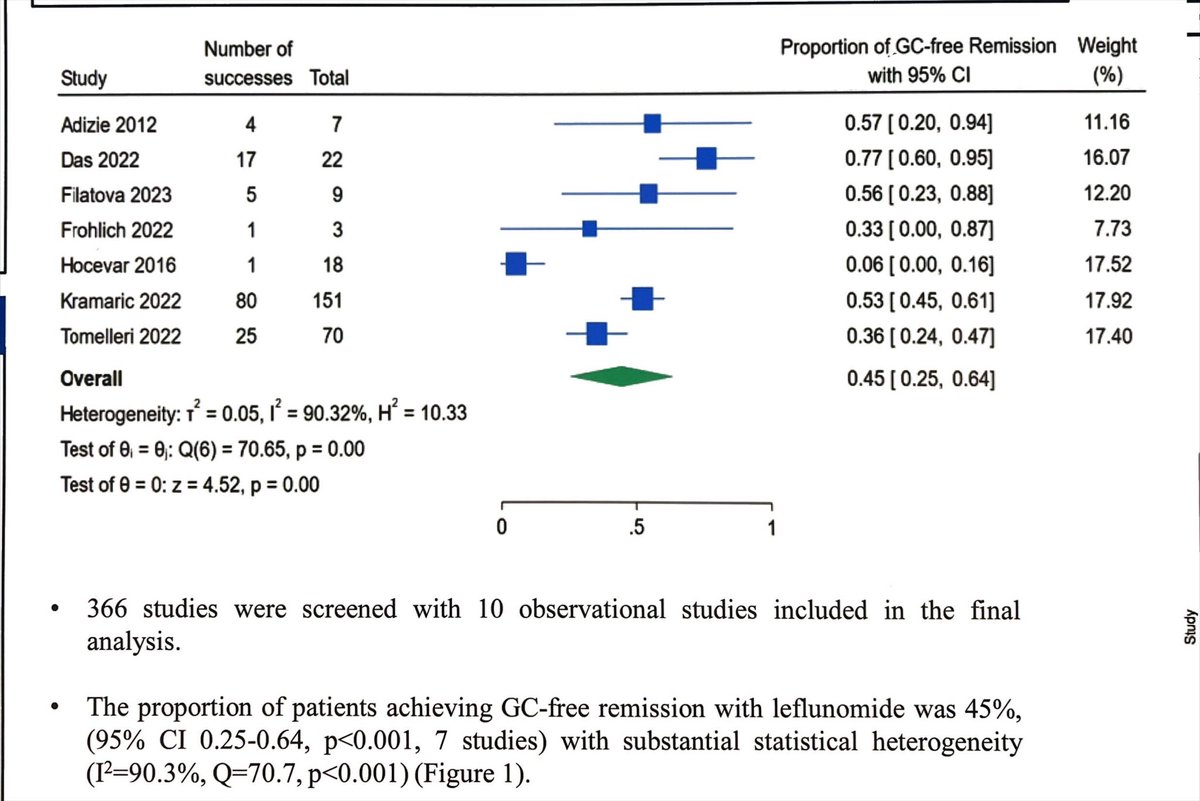
Leflunomide - a perennial question in GCA, that we still haven’t answered (as this meta-analysis shows), despite clear potential.
We are getting a clearer idea of the strengths/limitations of MTX in GCA, is it now time to get better clarity for LEF?
#ACR25 ABST0731 @RheumNow https://t.co/ycuvPx8Xdg
David Liew drdavidliew ( View Tweet)

Even when inflammation is quiet, fatigue often lingers. In 246 RA pts, 43% had significant fatigue—closely tied to PROMs, not labs. RAPID3 < 6 flagged the few truly “fatigue-free.”
Don’t overlook the VAS_Fatigue—it tells a real story.
@RheumNow #ACR25 Abstract# 0380
Jiha Lee JihaRheum ( View Tweet)
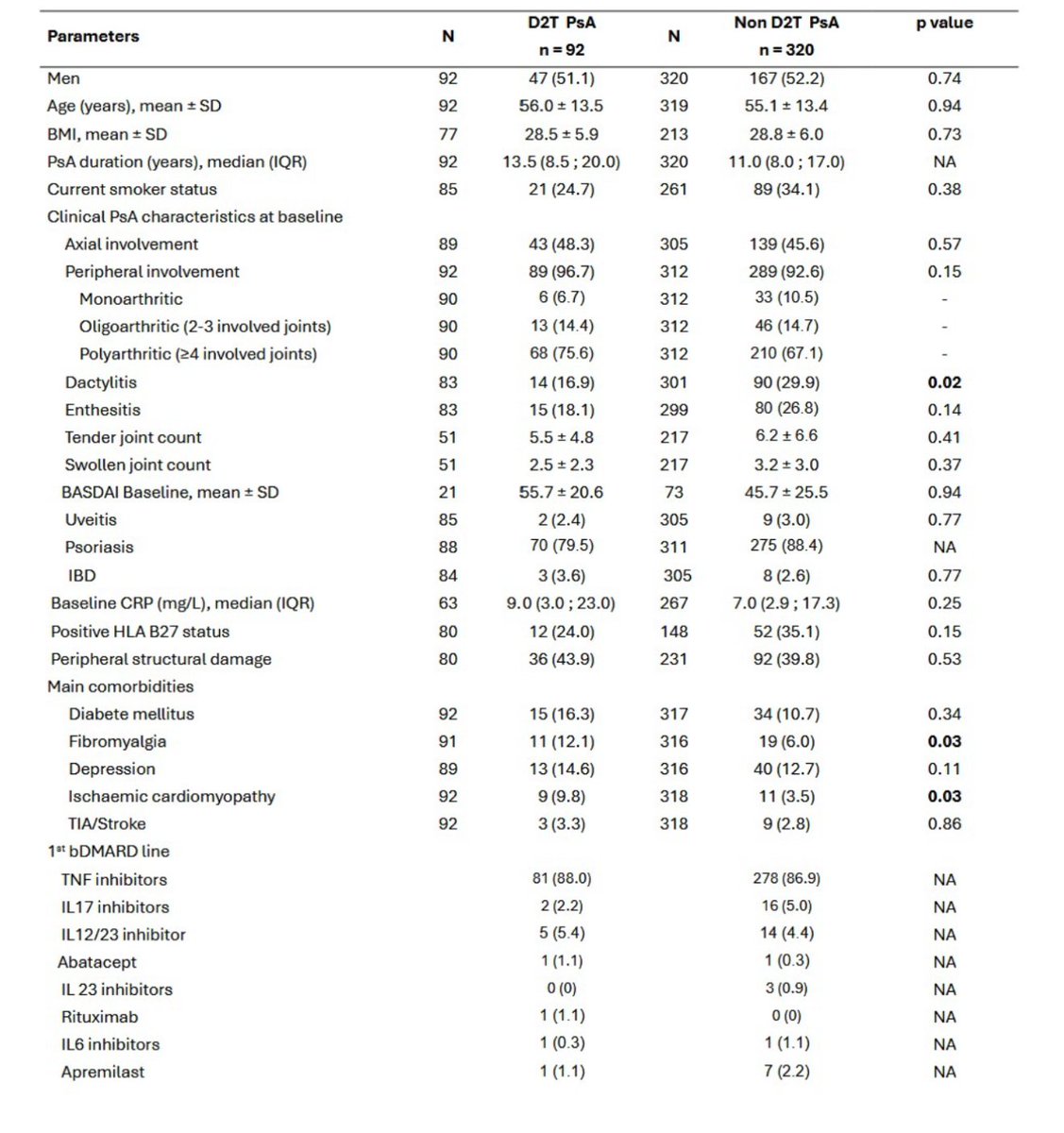
Retrospective study by Dr. Goudot et al. - pts w/D2T PsA had dactylitis, fibromylagia & ischemic cardiomyopathy
In multivariate analysis, FM & ischemic CM found to be RFs for D2T PsA
☝️Consider other dx if D2T PsA
#ACR25 @RheumNow ABS0566 https://t.co/FOXv6LbysZ
sheila RHEUMarampa ( View Tweet)
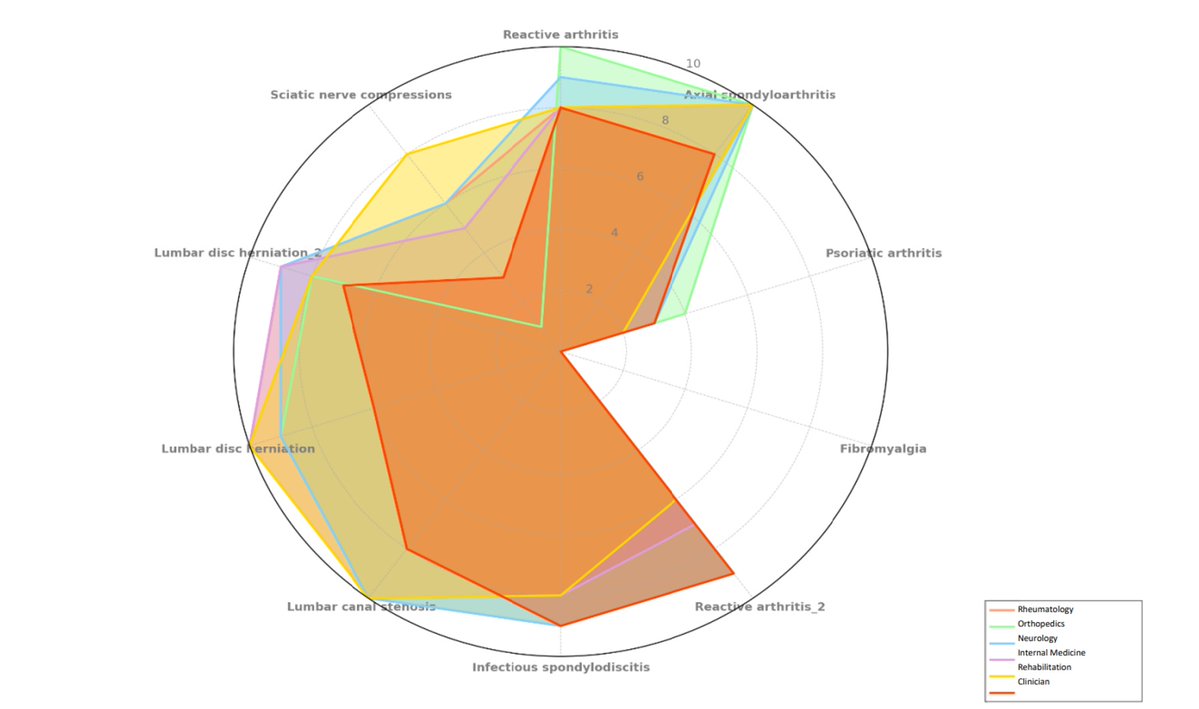
Using ChatGPT to diagnose axSpA, in 10 low back pain cases, it outperformed clinicians in diagnostic precision (75% vs. 60%) and sensitivity (85% vs. 80%), completing the task 5x faster. Specialty framing (e.g., Rheum vs Neuro) didn’t affect accuracy. Abstract 0530 @RheumNow https://t.co/YMMC4XIX9m
Antoni Chan MD (Prof) synovialjoints ( View Tweet)
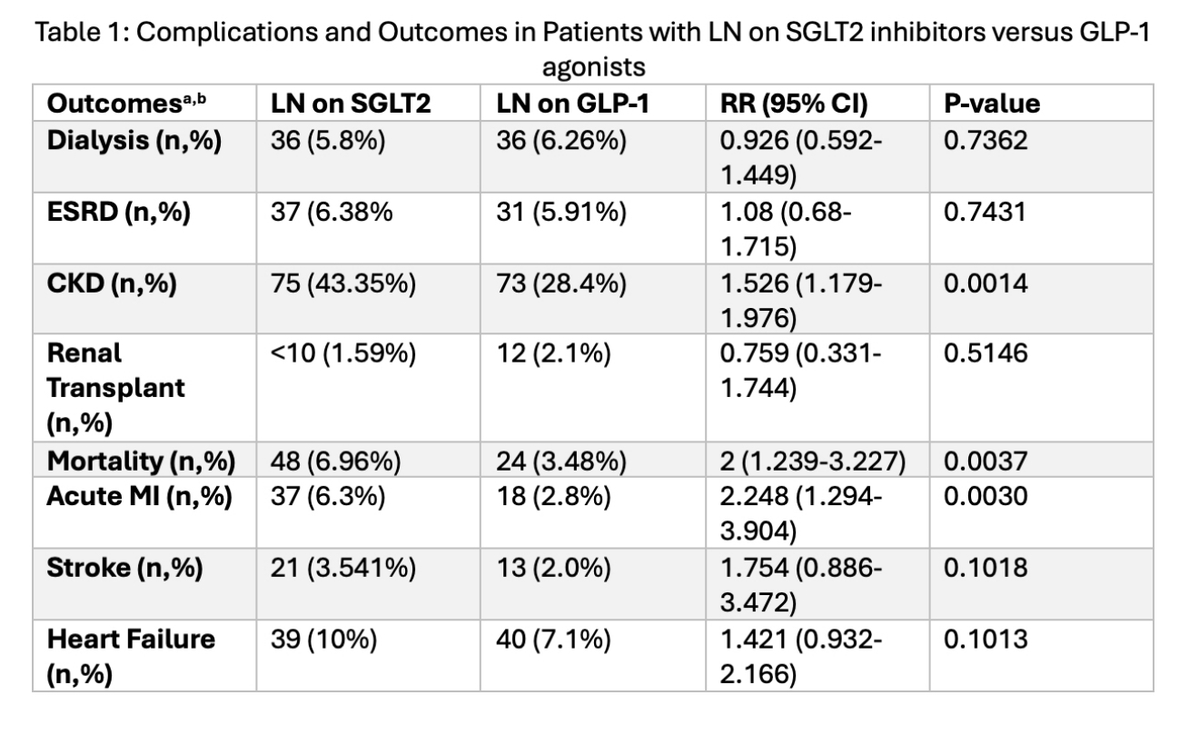
After propensity matching, this retrospective cohort study showed that #lupus nephritis patients on GLP-1 agonists had ⬇️ CKD progression, mortality & AMI risk vs. LN pts on SGLT2is.
Interesting data but what could explain it? Further studies are needed
#ACR25 @RheumNow Abs0841 https://t.co/FtsGbMDcCv
sheila RHEUMarampa ( View Tweet)
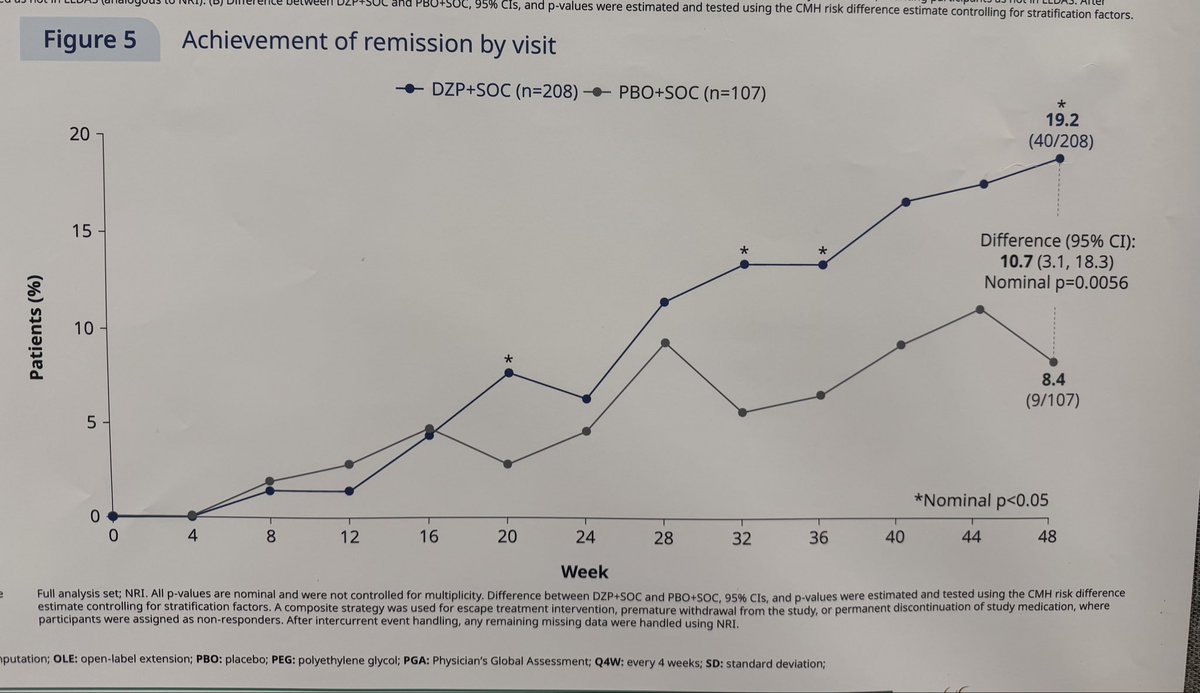
#ACR25 Abstr#645. Dapirolizumab, anti-CD40L-i met primary endpoint, BICLA in Phase 3 RCT non-renal SLE. Key secondary endpoints showed higher rates of & time in LLDAS and remission in DAP vs PBO. Meaningful targets re: damage accrual & better longterm outcomes @RheumNow https://t.co/IxesXhi34R
Links:
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)
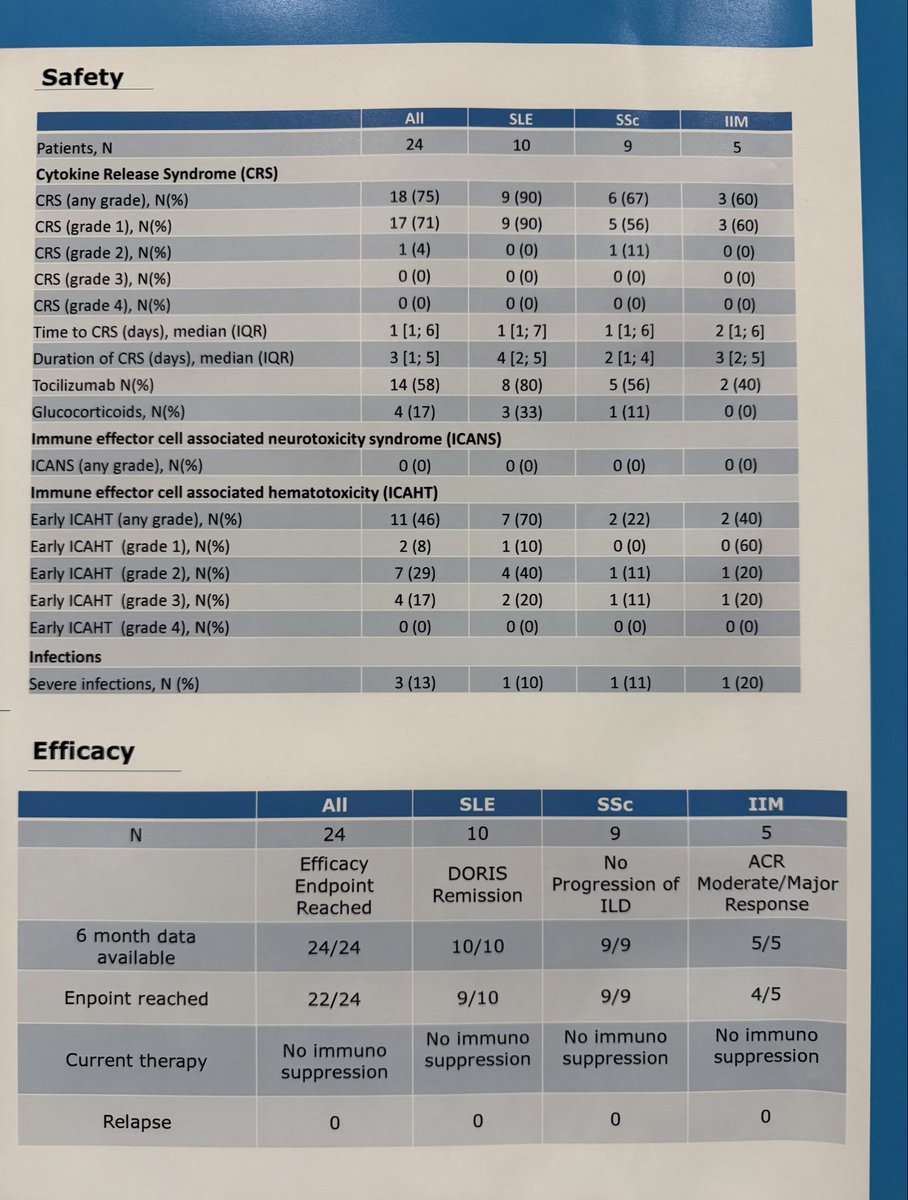
#ACR25 Abstr#641 Phase 1/2 CASTLE Basket Trial fully recruited N=24 (10 SLE, 9 SSc, 5 IIM). Short-term data of autologous CD19-CAR T
Zorpocabtagene-autoleucel/MB19.1:
- 22/24 high degree efficacy
- no relapse
- no high grade CRS/ICANS
*Need larger Phase prior approval @RheumNow https://t.co/sp1nieeCvl
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)

Real-world JAK persistence in RA is low: <30% at 2 years across Medicare & Marketscan data (n≈20K). Baricitinib lowest persistence; TOF & UPA slightly higher. Oral convenience hasn’t solved long-term adherence. Why? Safety, cost, access?
@RheumNow #ACR25 A#0369
Jiha Lee JihaRheum ( View Tweet)
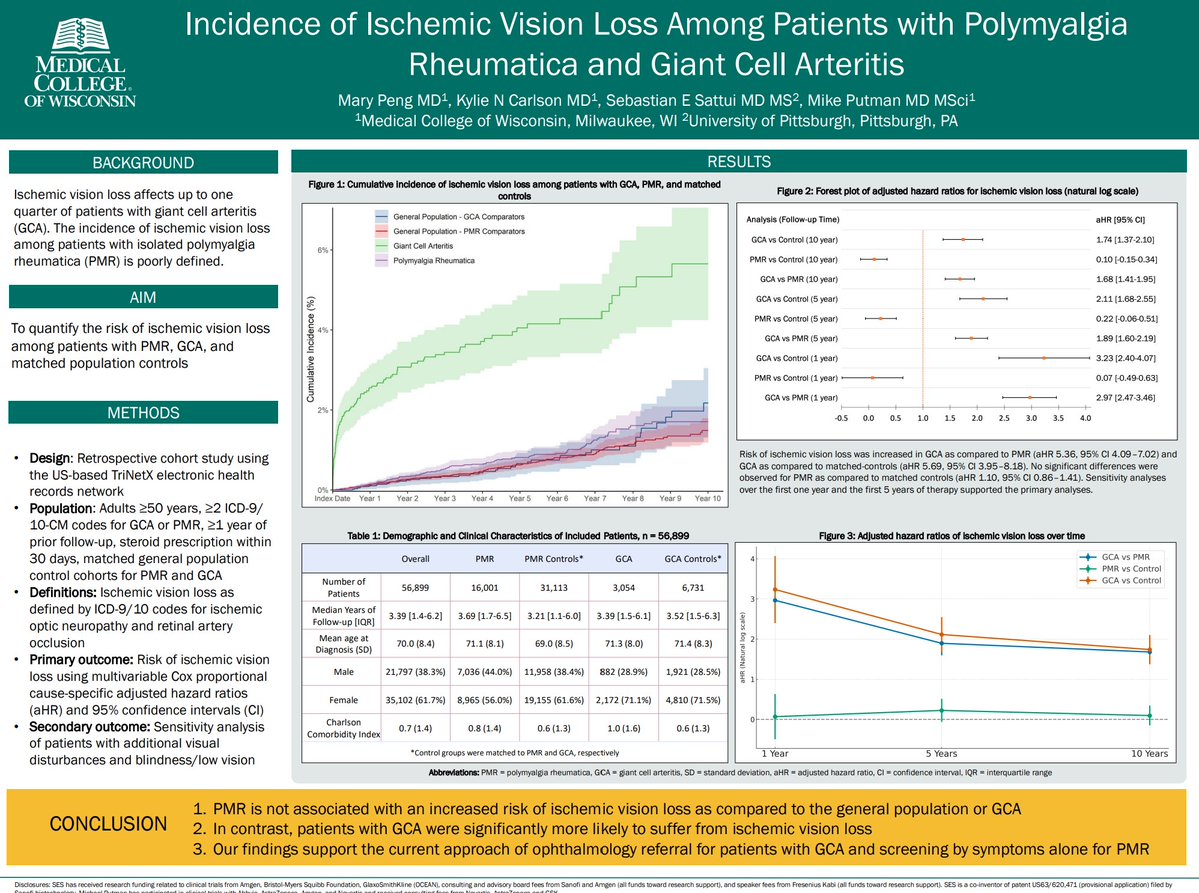
Project from my group & rheum-bound resident Mary Peng!
As expected, ischemic vision loss for GCA compared to PMR (HR 5.4) and gen pop (HR 5.6) 😬
NO difference for PMR vs gen pop (aHR 1.10, 95% CI 0.86–1.41)
Reassuring for pts w/PMR!
#ACR25 @RheumNow Abstr#0753 #ACRBest https://t.co/HKzc7OOzmP
Mike Putman EBRheum ( View Tweet)

Only 32% of RA pts were satisfied with current txs. In a discrete choice experiment of 354 US pts, many preferred a vagus nerve stimulator over another biologic/JAKi, especially those with prior tx failures. Cost, function, and fatigue drove preferences.
@RheumNow #ACR25 A#0370
Jiha Lee JihaRheum ( View Tweet)

Icotrokinra, a first-in-class targeted oral peptide IL-23R inhibitor, cleared scalp (66%), genital (77%), and overall skin (57%) PsO vs placebo at Week 16. Basket trial design enabled targeted evaluation. Safety profile favorable. Abstract 0555 @RheumNow #ACR25
Antoni Chan MD (Prof) synovialjoints ( View Tweet)
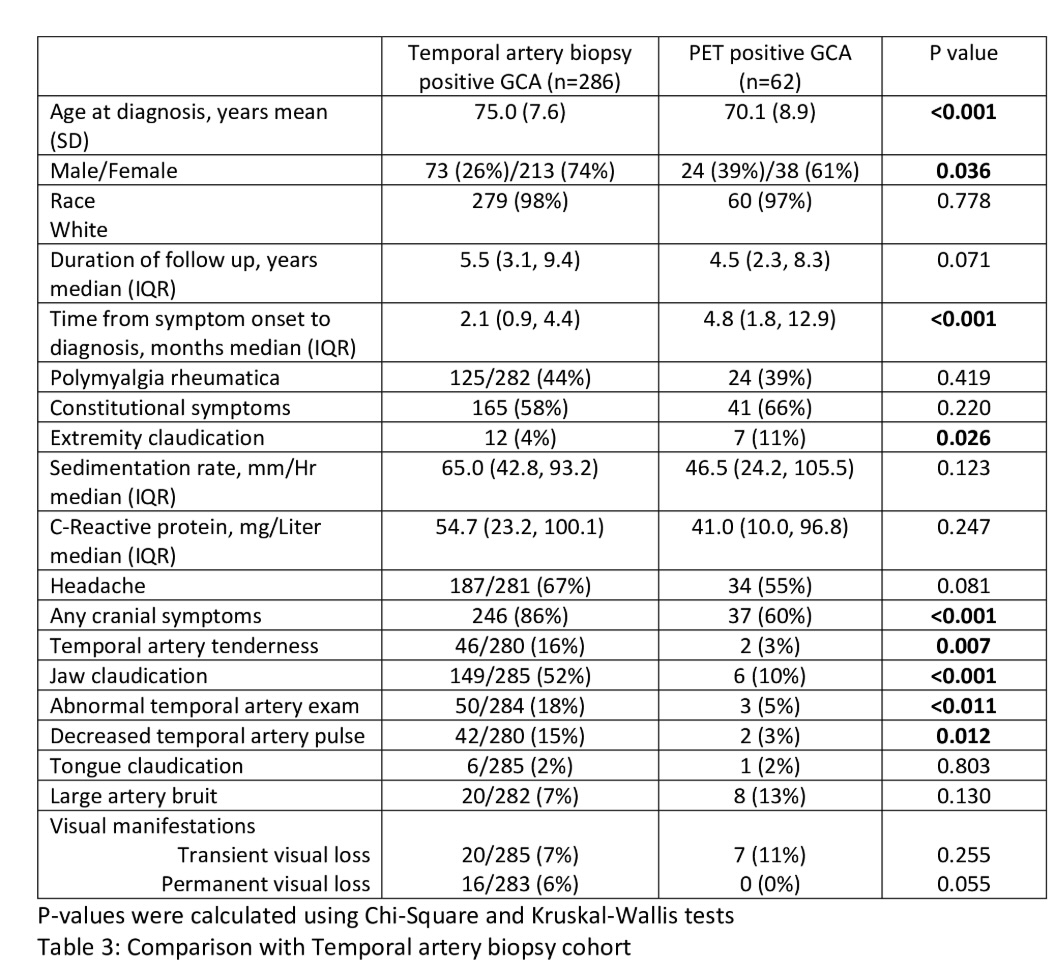
Phenotypes of GCA:
Pt dx by PET compared to TA biopsy were:
- younger
-longer disease before dx
-more frequent extremity claudication (still overall low)
-less cranial / TA sx
@RheumNow #ACR25 Abstract 0759 https://t.co/ilwa3eYmRq
Brian Jaros, MD Dr_Brian_MD ( View Tweet)
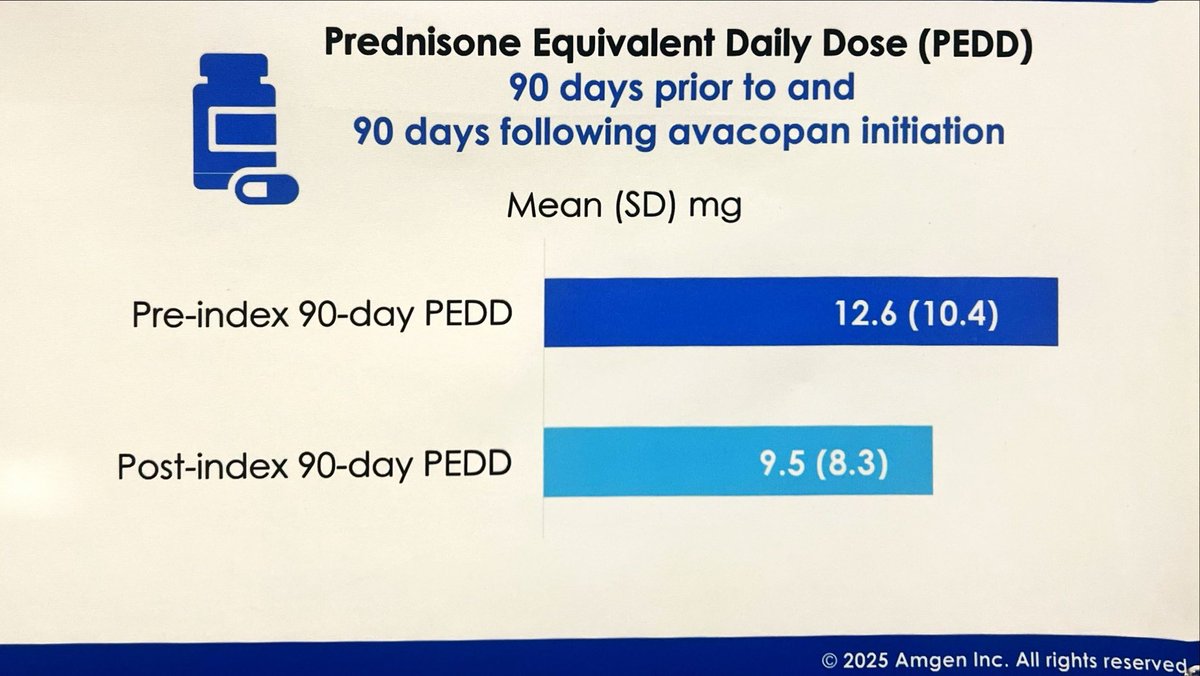
Avacopan in the real world: we need to be braver
If we want to get steroid-sparing benefit in AAV, we need to trust it
claims data shows modest PNL reduction with avacopan
some manifestations are harder to taper in, but others we just need to be bolder
#ACR25 ABST0726 @RheumNow https://t.co/51nGdpR2db
David Liew drdavidliew ( View Tweet)

Can the blood reflect what’s happening in the joint? In early RA, 11 synovial proteins mirrored serum levels, forming an inflammatory signature linked to disease activity and csDMARD response. High-signature pts had stronger treatment responses (AUC>0.8)
@RheumNow #ACR25 A#0070
Jiha Lee JihaRheum ( View Tweet)
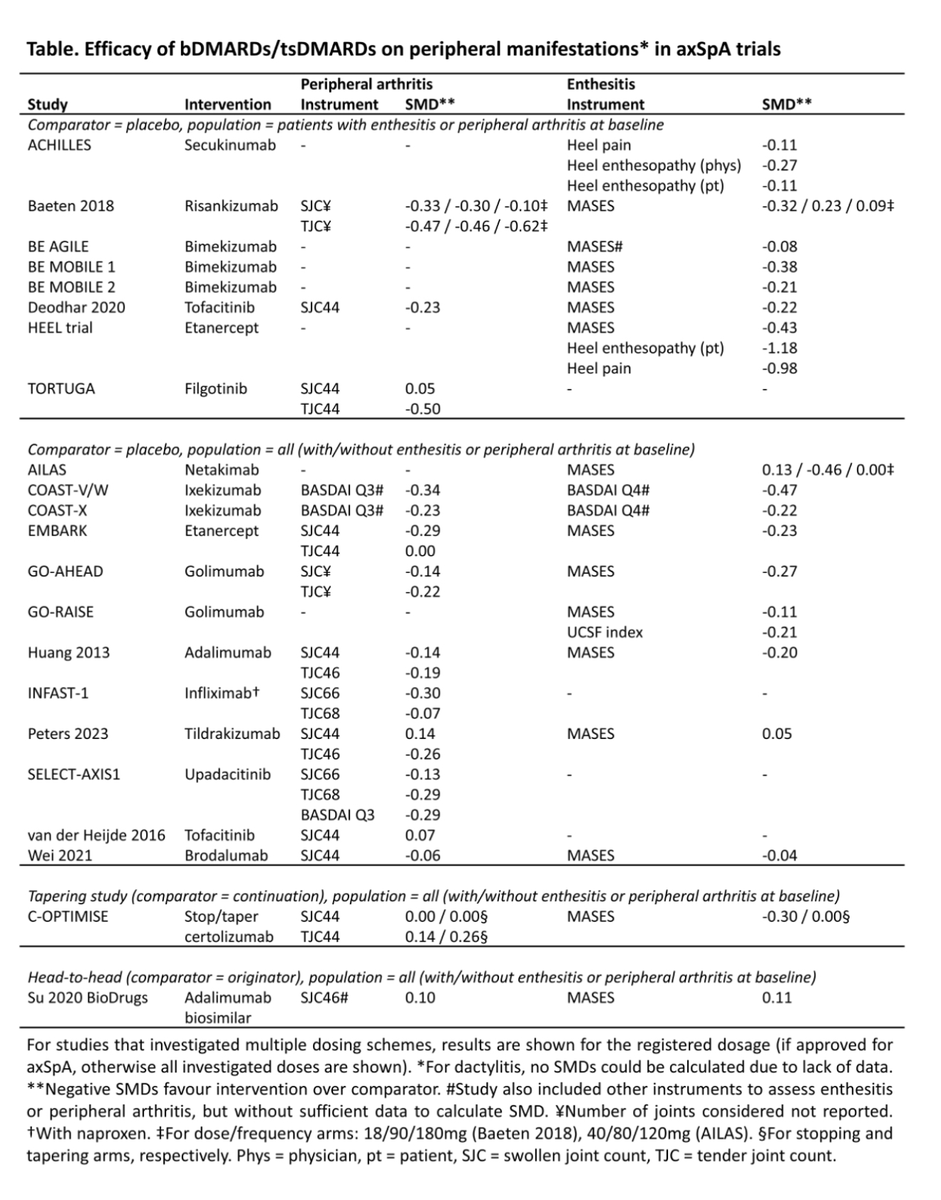
A systematic review by Dr. SRamiro et al evaluated efficacy of b/tsDMARDs on peripheral axSpA
Majority on bDMARDs (TNFi, n=24; IL-17, n=13)
Most of the SMDs were small --> b/tsDMARDs showed small to moderate effects on peripheral arthritis & enthesitis.
#ACR25 @RheumNow Abs0588 https://t.co/yGXcqhZ1Uv
sheila RHEUMarampa ( View Tweet)
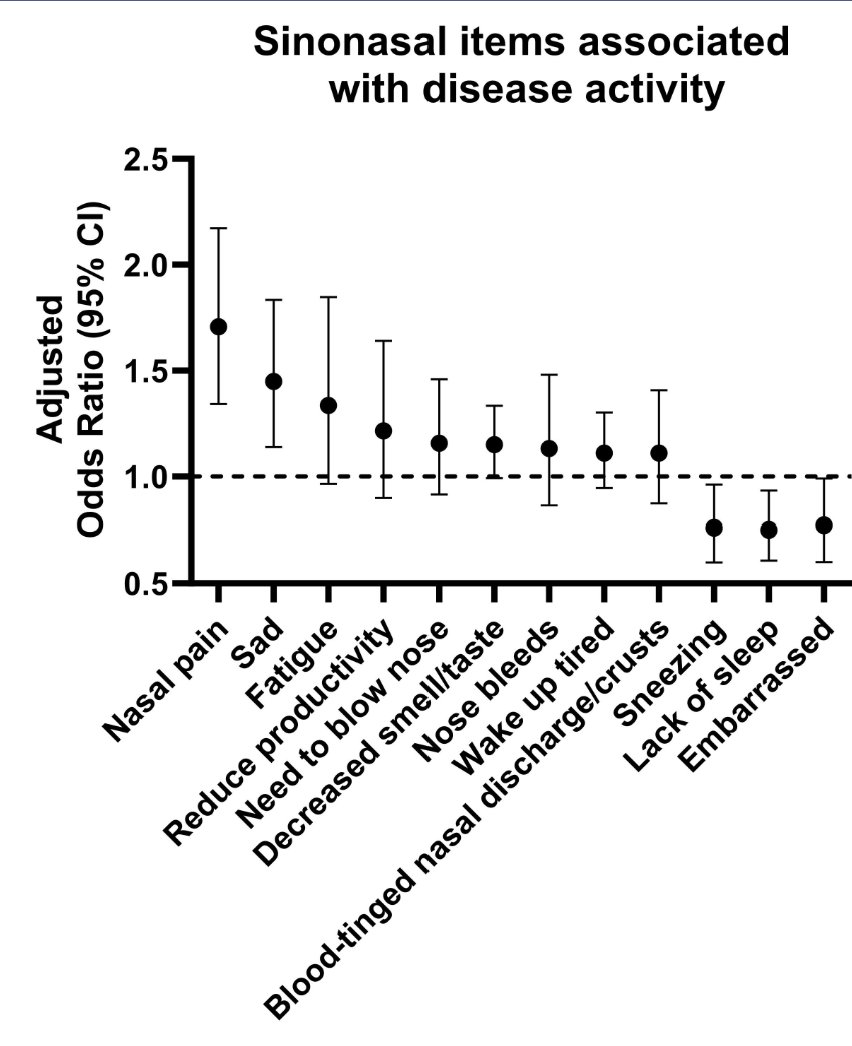
Cool study using vasculitis patient-powered reserach network (VPPRN)
"SNOT-22" tool captures sinonasal symptoms; associated with disease activity (expected) but also worse among pts even in remission
Very important impacter of QOL in AAV
#ACR25 @RheumNow Abstr0725 https://t.co/NQBW8VnYFS
Links:
Mike Putman EBRheum ( View Tweet)
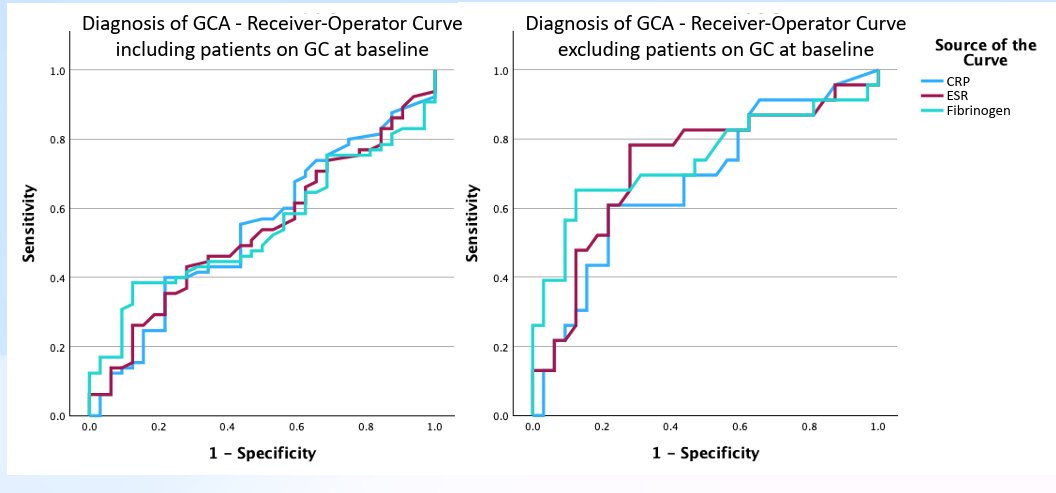
Miller et al. Fibrinogen for GCA diagnosis and flare identification. Similar to ESR/CRP in diagnosis. For flares, lower sensitivity (79%) but better specificity (96%) @RheumNow #ACR25 Abstr#750 https://t.co/SIkAkEeYh1
Richard Conway RichardPAConway ( View Tweet)



