All News
In Giant Cell Arteritis, Hospital Admission Is Bad News
One-third of patients admitted with giant cell arteritis (GCA) were rehospitalized within 6 months, largely because of complications potentially related to corticosteroid therapy, researchers found in a retrospective cohort study.
Read ArticleACP Recommends Bisphosphonates as Initial Therapy for Osteoporosis
The American College of Physicians (ACP) has issued an update of its guideline with clinical recommendations for treatments of primary osteoporosis and low bone mass in adults.
Read Article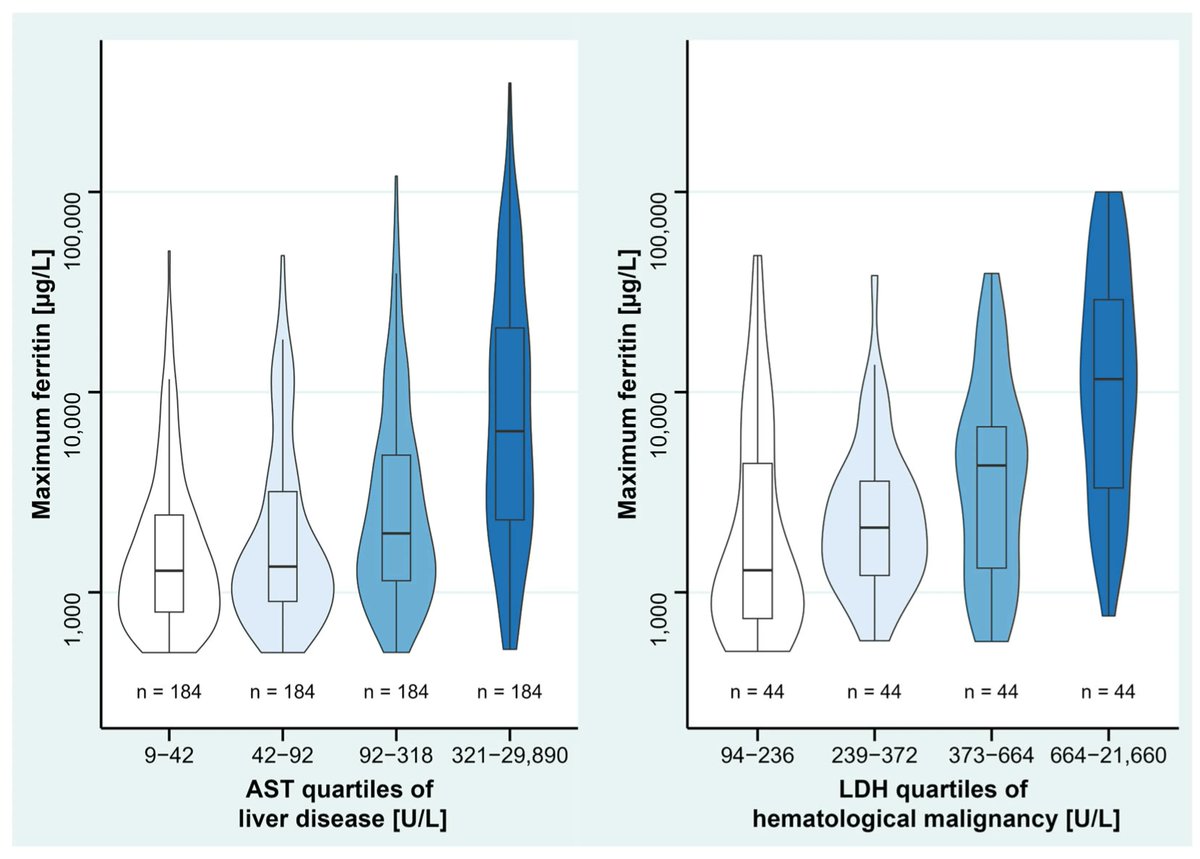
Differential Dx of hyperferritinemia (≥500 μg/) in critically ill ICU pts includes: sepsis, liver disease/failure, hematological malignancy, HLH. Single center study of 2583 patients https://t.co/yuqJcyLIP5 https://t.co/vHLqJGP4mv
Links:
Links:
Links:

Links:


Links:
Dr. John Cush RheumNow ( View Tweet)
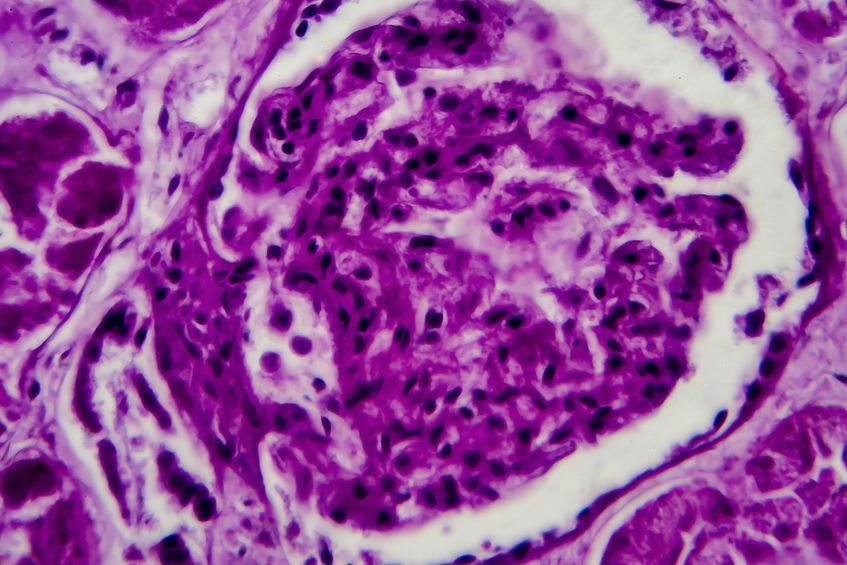
Links:

Links:

Links:


Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)
Links:

Dr. John Cush RheumNow ( View Tweet)
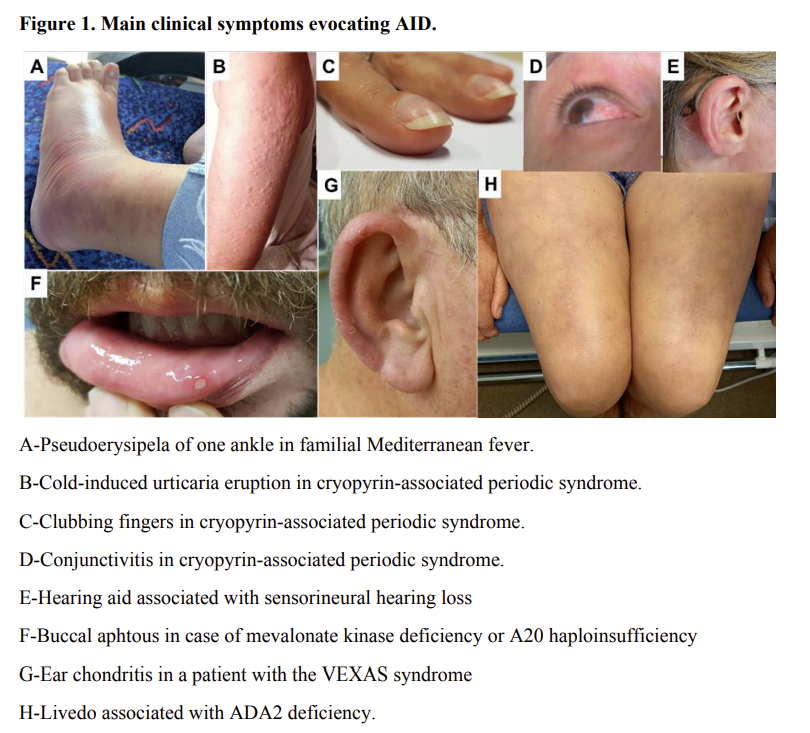
Links: