All News
Is PMR the Next Indication for Tocilizumab?
About half of patients with steroid-dependent polymyalgia rheumatica (PMR) were able to get off steroids altogether when they were started on tocilizumab (Actemra) infusions, which also improved disease control in most cases, a randomized phase III trial showed.
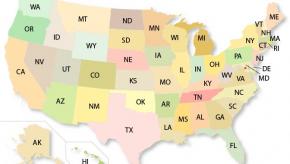
Read ArticleACR's State-by-State Report Cards for Rheumatic Disease
New report examines access, affordability, and activity and lifestyle factors in all 50 states and the District of Columbia, evaluating how easy it is to live with a rheumatic disease in your state. No state scored an "A" or "F", and only two states improved their 2018 grades.
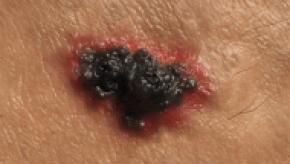
Read ArticleDoes Methotrexate Use Lead to Melanoma?
A systematic review suggests that low-dose methotrexate (MTX) use is associated with an increased melanoma risk, but the absolute risk increase could be considered negligible.
Read ArticleFirst In Class, TYK2 Inhibitor FDA Approved for Psoriasis
Deucravacitinib (Sotyktu), a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, is the only approved TYK2 inhibitor worldwide and the first innovation in oral treatment for moderate-to-severe plaque psoriasis in nearly 10 years.
Read ArticleDoes Fibromyalgia Need B12? (9.9.2022)
Can we predict the bad outcomes? Like when ITP evolves into SLE; or when psoriasis will develop arthritis; or if Sjogren's will develop lymphoma? Let's dive in and review these journal reports and this past week's news from RheumNow.com.
Read ArticleDoes Dendritic Cell Targeted Therapy Work in SLE?
A phase 2 trial using litifilimab, a humanized monoclonal antibody binding to blood dendritic cell antigen 2 (BDCA2), demonstrated clinical efficacy in adults with systemic lupus erythematosus (SLE).
Read ArticlePirfenidone Potential in RA-Related Lung Disease
Rheumatoid arthritis patients with existing interstitial lung disease (ILD) had less decline in lung function when receiving the antifibrotic agent pirfenidone (Esbriet) relative to placebo in a randomized trial, researchers reported here and in a simultaneous journal publication.
Read ArticleGlobal Trends Informing the Future of Rheumatoid Arthritis
Finkh et al report on the prevalence and interesting trends in rheumatoid arthritis (RA), including its higher prevalence in industrialized countries and declining disease severity over time.
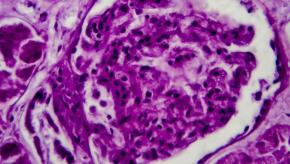
Read ArticleVoclosporin Efficacy and Safety in Lupus Nephritis
An integrated analysis of two pivotal trials of voclosporin, a calcineurin inhibitor, in lupus nephritis patients saw significant improvement in complete renal responses (CRR) at one year.
Read ArticleGoofy But True
Dr. Jack Cush discusses declining survival rates in the USA, FDA approvals of new COVID subvariant boosters and other odd and possibly true new research reports from the past week on RheumNow.com.
Read ArticleGenetic Testing for Autoinflammatory Disease
Not all patients with periodic fevers fit neatly into diagnostic categories. Some can be diagnosed as Still’s disease (based on criteria) while others can be classified as autoinflammatory diseases (AID) and some may be unclassifiable, clinically or genetically.
Read ArticleFDA Approves BA.4 and BA.5 Omicron COVID Booster Vaccines
Today the FDA authorized the updated Omicron subvariant (BA.4 and BA.5) COVID-19 booster shots manufactured by Pfizer and Moderna; with an anticipated ship/start date of early September 2022. The BA.5 subvariant accounts for more than 88% of U.S. infections.
Read ArticleNICE Guidelines on Gout Diagnosis and Management
NICE (UK) has systematically reviewed current medical evidence and delivered a set of recommendations with consideration of cost effectiveness.
Read ArticleOlokizumab, another IL-6 Inhibitor for RA
This week's NEJM has published the efficacy results of a large phase 3 trial of olokizumab, a humanized monoclonal antibody that directly targets IL-6 in patients with rheumatoid arthritis.
This is in contrast to two other marketed IL-6 inhibitors (sarilumab, tocilizumab) that bind to the IL-6 receptor.
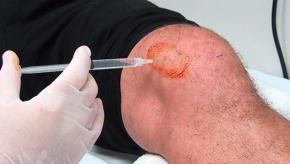
Hyaluronic Acid Knee Injections Equivalent to Placebo
In the United States, where over $300 million is spent annually on intraarticular hyaluronic acid injections, yet another study shows such therapy to be no better than placebo.
Read ArticleAxial Psoriatic Arthritis and Ankylosing Spondylitis with Psoriasis
A cohort analysis from Toronto suggests that axial psoriatic arthritis (PsA) is distinctly different from axial ankylosing spondylitis (AS) with psoriasis.
Read ArticleManaging JDM with Calcinosis
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow and discusses a case of refractory juvenile dermatomyositis with calcinosis.
Read ArticleJUNIPERA Study - Secukinumab in Juvenile PsA & ERA
JUNIPERA study evaluated secukinumab (SEC) in children with enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) and was found to be safe and effective in patients with active ERA and JPsA who previous failed to respond to conventional therapy.
Read ArticleThe Great Unknowns (8.5.2022)
Dr. Jack Cush reviews the news, FDA approvals, journal articles from the past week on RheumNow; plus viewer questions. This week great hopes for vitamin D, the great unknows of CSA and the great big mess that is the gout.
Read ArticleACR Updated Guideline on Vaccinations for Rheumatic Patients
The ACR has posted a new ACR Clinical Practice Guideline Summary providing recommendations on the use of vaccinations for children and adults with rheumatic and musculoskeletal diseases (RMDs).
Read Article