All News
What is JAKne? (5.6.2022)
Dr. Jack Cush reviews the news, journal reports, FDA actions, new side effects and changing prevalence of Gout.
Read ArticleLack of Research Stymies Uptake of Medical Marijuana for Rheumatic Pain
A new review article from CreakyJoints finds that there has been limited progress in understanding the potential of cannabis based therapies for the treatment of pain associated with rheumatic conditions in the past five years because of a lack of standardization of clinical research and barriers to conducting research due to existing federal and state regulations.
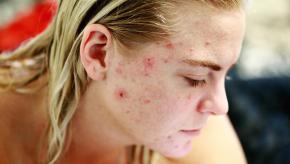
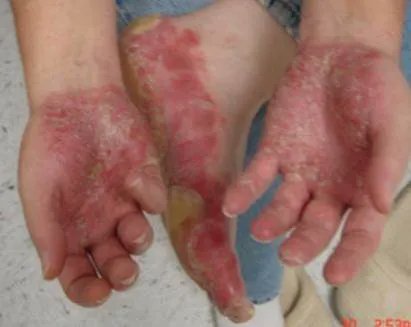
Read ArticleAcne with JAK Inhibitors
JAK inhibitors have become enormously popular and while their side effect profile has been delineated and reviewed, little mention is made of acne – an adverse event that may affect up to one-quarter of patients taking JAK inhibitors (JAKi).
Read ArticleBSR Guideline on Idiopathic Inflammatory Myopathy
The British Society of Rheumatology (BSR) has published evidence-based guidelines for idiopathic inflammatory myopathy (IIM) affecting juvenile and adult-onset disease. This rare condition has an estimated prevalence of 5-15 per 100,000 persons.
Read Article
Links:

Links:

Dr. John Cush RheumNow ( View Tweet)

Links:
Links:
Links:
Links:

Links:
Dr. John Cush RheumNow ( View Tweet)
Links:
Links:

Links:
Dr. John Cush RheumNow ( View Tweet)
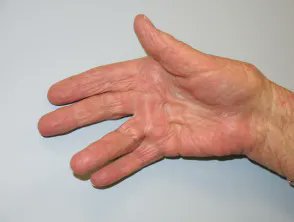
Links:

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)