All News
TNR - Controversies in PsA
Held on April 19, 2022, Tuesday Night Rheumatology: Controversies in PsA, featured a lively and informative panel discussion, moderated by Dr. Jack Cush.
The PsA Expert panelists included:
Read ArticleFaith in Exercise Benefit Boosts Actual Benefit in SpA
Patients with axial spondyloarthritis (SpA) got more bang for their exercise buck in a randomized trial when they had good opinions about their workout program, with better persistence with exercise at 1 year relative to a control group, researchers said.
Read ArticleDietary Improvement in Psoriatic Arthritis
The results of the DIETA trial showed that dietary changes may control psoriatic arthritis (PsA) disease activity, independent of weight loss.
Read Article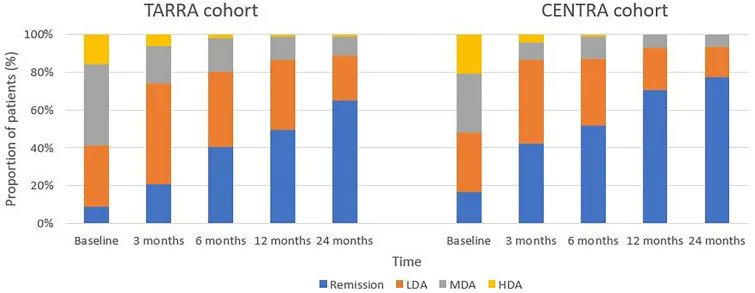
Links:

Links:

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)
Links:
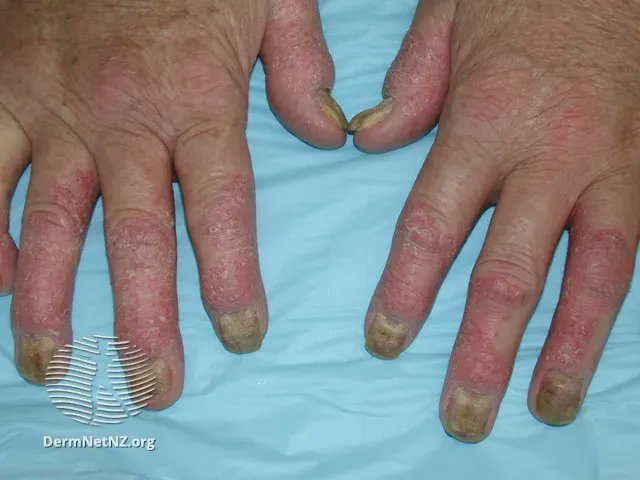
Links:
Links:

Tanmayee Bichile, MD BichileT ( View Tweet)

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)
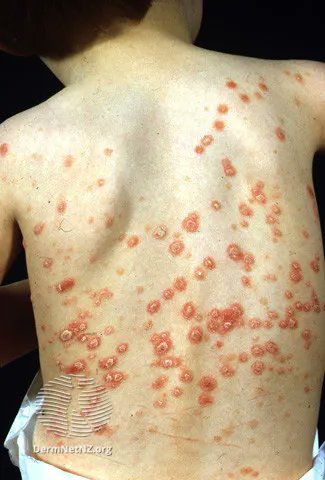
Links:

Links:

Links: