All News
#OP0220
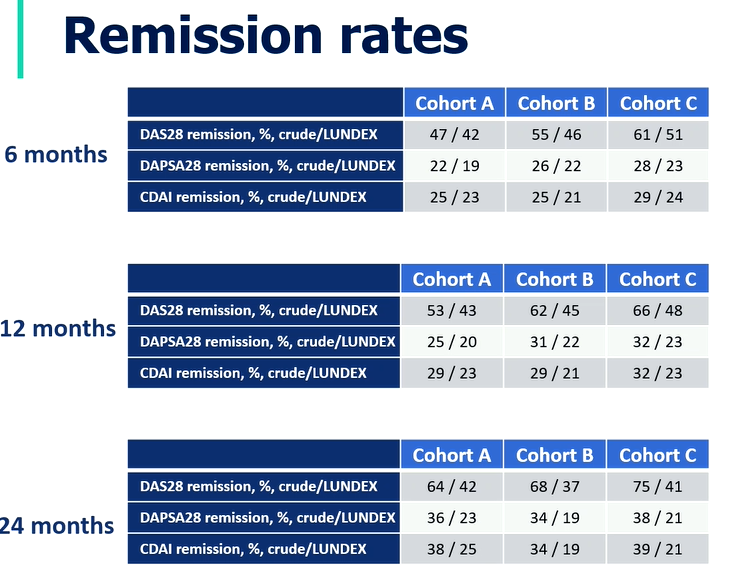
EuroSpA 17 European registries
17452 bionaive PsA Patients 1999-2018:
BL age, disease duration, disease activity decreased 99-18
- disease activity progressively decreased on treatment
- At 6M REM rates higher in recent years, 24M REM rates similar
#EULAR2021 @RheumNow https://t.co/gZ4tOpRLIf
Paul Studenic Stiddyo ( View Tweet)
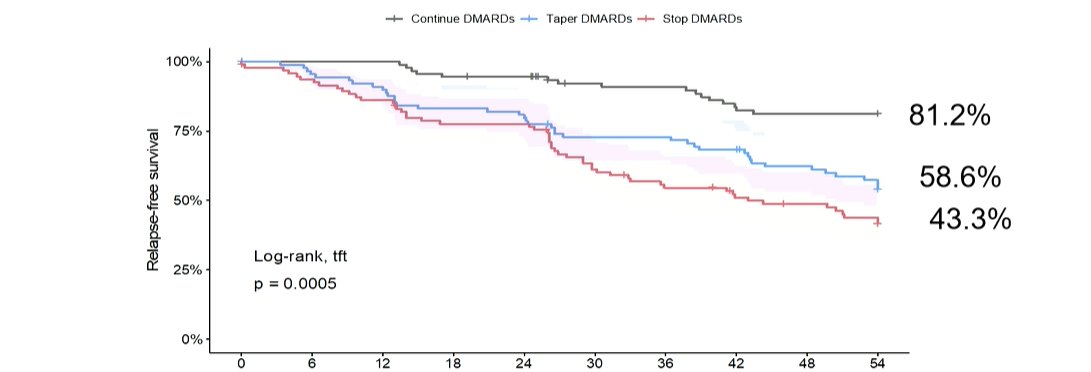
OP0318 What to consider when tapering DMARDs
RETRO – RA pat with DAS<2.6 for >=6months randomised to 3 strategies: remain – taper to 50% - taper then STOP
HR for flare 3|4.3 for TAP|STOP
Remission depth, disease duration and RF/ACPA as risks to consider
#EULAR2021 @RheumNow https://t.co/SR4NDKKrGN
Paul Studenic Stiddyo ( View Tweet)

#POS0660
Integrated data analyses of the DARWIN, and FINCH studies (7 in total) on #filgotinib and #statin use:
> Around 10% of 4057 RA patients used statins at baseline
> Muscle related AE were rare with no clear relation to filgotinib
#EULAR2021 @RheumNow
Paul Studenic Stiddyo ( View Tweet)
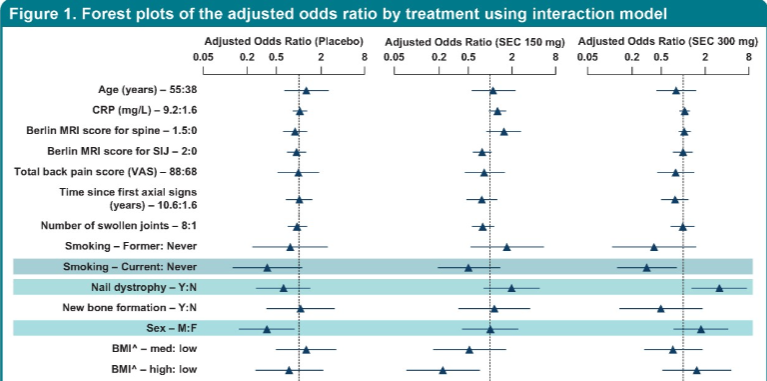
#POS0930
@XBaraliakos investigated baseline predictors differential treatment effect on ASAS20 response at week 12 of 473 ax-PsA using MAXIMISE trial data (#Secukinumab)
-> Only nail dystrophy
OR: 3 (150mg)
OR: 5 (300mg)
🤔But why?
#EULAR2021 @RheumNow https://t.co/srb6jGgBqe
Paul Studenic Stiddyo ( View Tweet)
#EULAR2021 live might close but there is still on demand until the 4th of July.
Interested in my poster: showing that quality of life in at-risk for #RA individuals is reduced but still differ from RA.. have a look at #POS1441
New insights in "pre-disease phase"
@RheumNow https://t.co/xtyEgElYkX
Paul Studenic Stiddyo ( View Tweet)

What kind of trials would help you in making a decision which DMARD to choose if a patient is not meeting #T2T goal?
#EULAR2021 @RheumNow
Paul Studenic Stiddyo ( View Tweet)
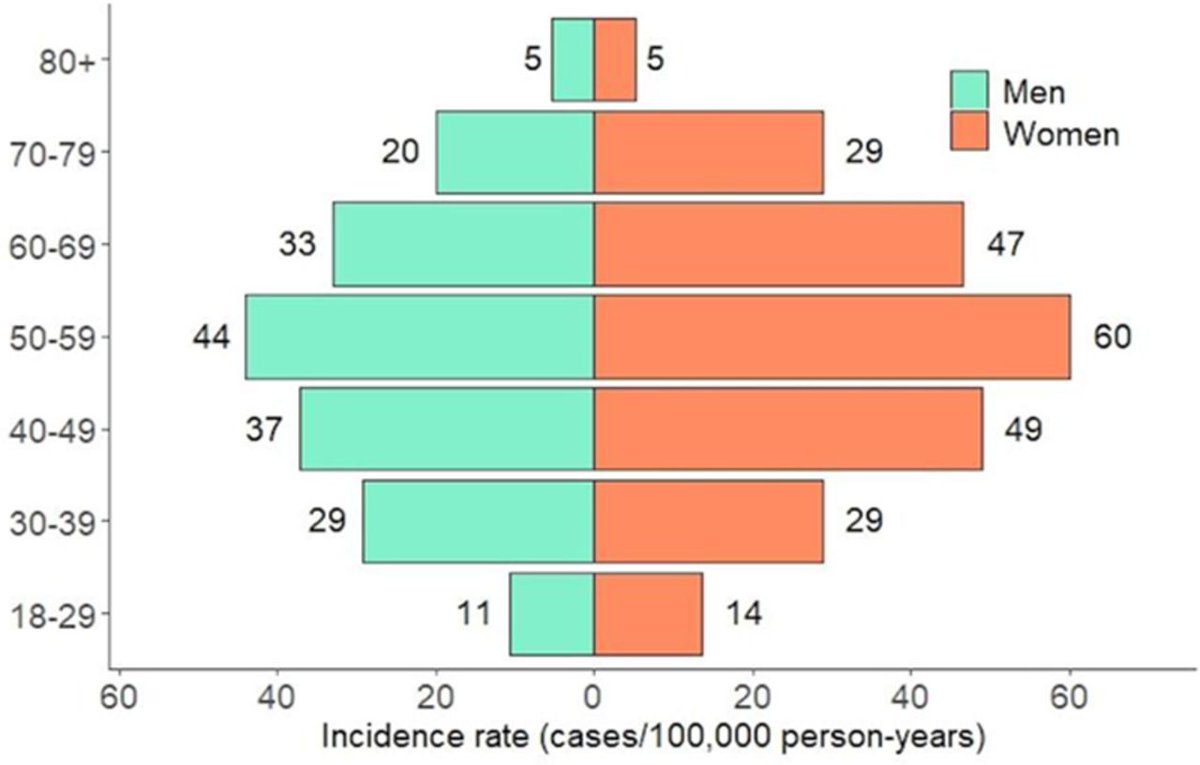
Norwegian Cardio-Rheuma register review of PsA incidence 32/100,000 pyrs, 28 among 🚹 & 35 among 🚺,highest in 50-59 yo pts. MTX initiated >50% PsA cases w/in 1 yr from index date, 19% used biologic DMARDs w/in 2 yrs. Abstract #POS1041 #EULAR2021 @RheumNow https://t.co/2PGVfeoFV9 https://t.co/CT9WTzcdcc
Dr. Rachel Tate uptoTate ( View Tweet)
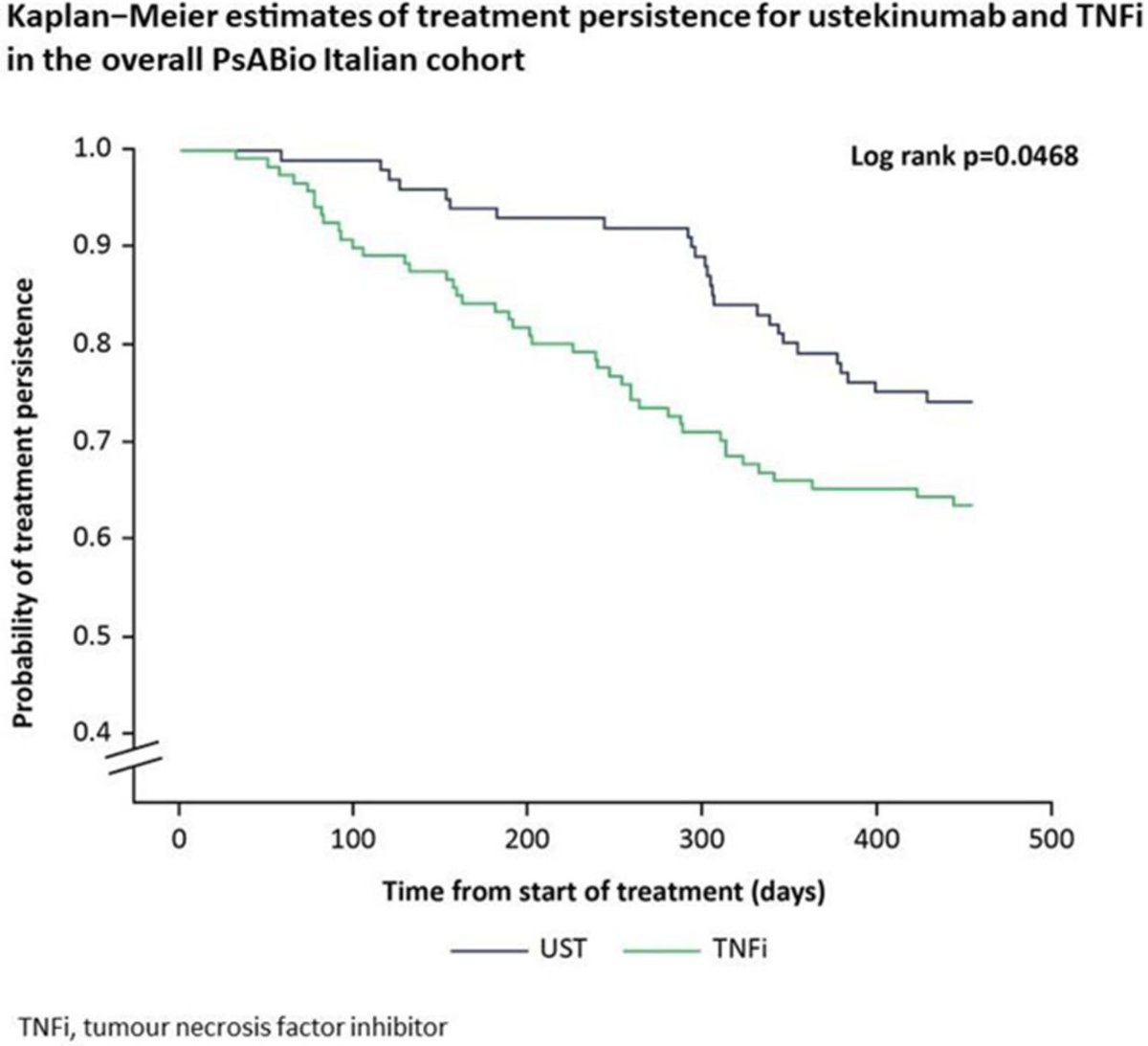
Italian PsABio cohort study: UST ⬆️ overall persistence vs TNFi, especially in females, in monotherapy, BMI <25 or >30 kg/m2, & UST as 2nd-line tx. At 1 year, both UST & TNFi pts showed similar cDAPSA & MDA/VLDA. Abstract #POS1021 #EULAR2021 @RheumNow https://t.co/DGRXmPiTiD https://t.co/s7zMuSBEWt
Dr. Rachel Tate uptoTate ( View Tweet)
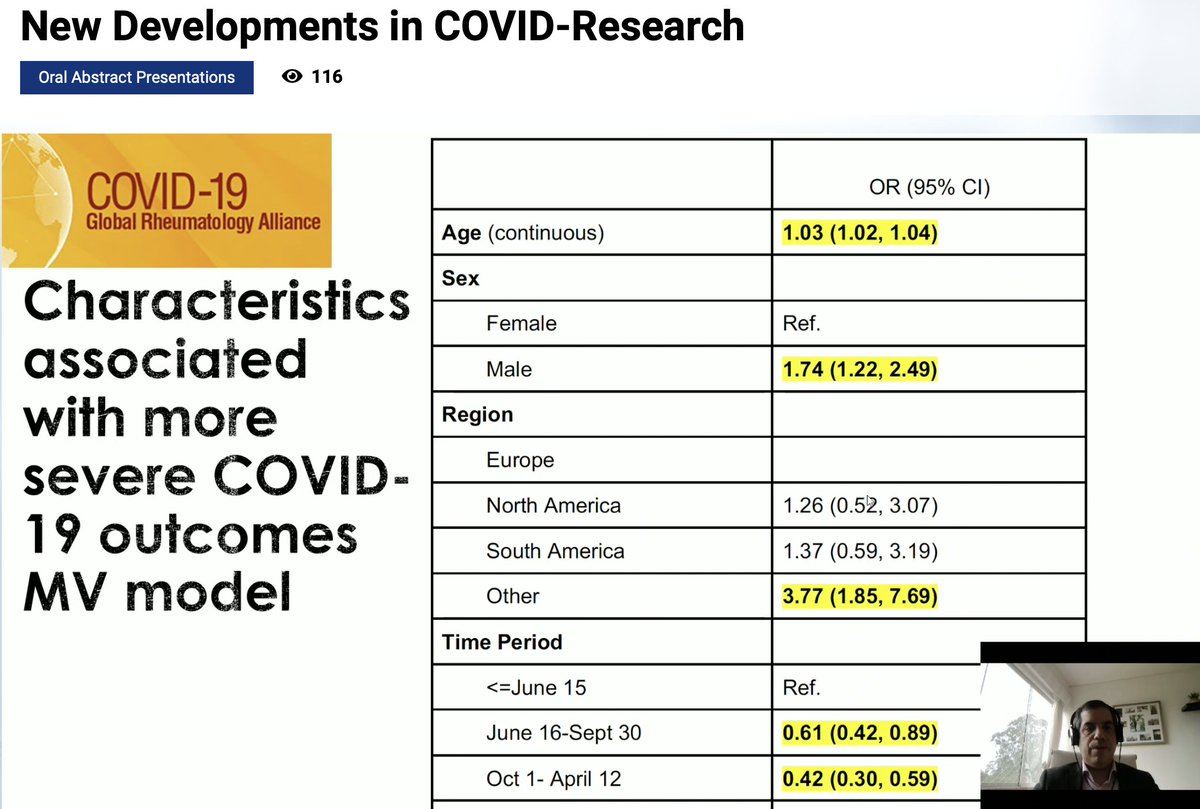
More data from @rheum_covid presented by @mugartegil showing prognostic factors in patients with SLE & COVID-19
Factors related with poor prognosis:
-Male, Age, Comorbidities
-Time period (1st wave)
-GC doses
-Disease activity
- No enough data for RTX or Beli
#EULAR2021
#LUPUS https://t.co/ts8QsyR5KA
José A. Gómez Puerta DrGomezPuerta ( View Tweet)

What is the highest level reaction you have seen to COVID vaccine? @RheumNow #EULAR2021
Richard Conway RichardPAConway ( View Tweet)
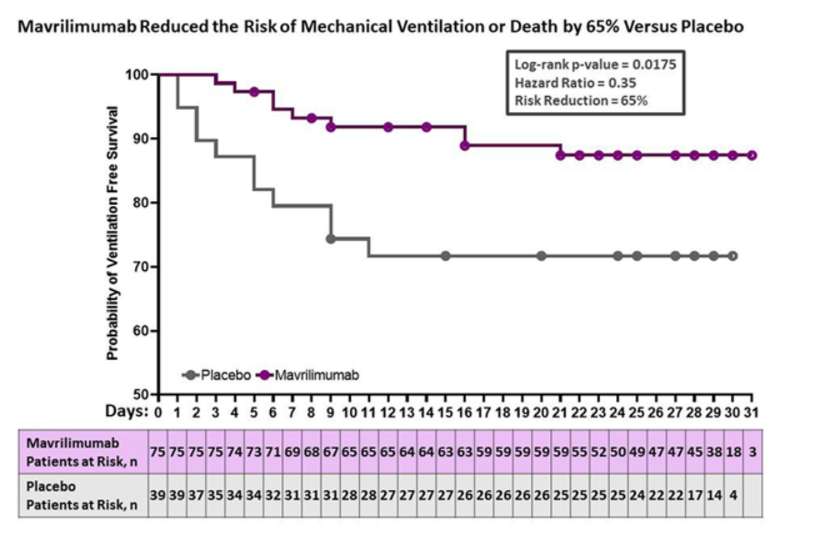
Dr Pupim presents data on GM-CSF inhibitor mavrilimumab to treat non-ventilated COVID-19 with systemic hyperinflammation 65% ⬇️ ventilation/death day 29 (p=0.02) 61% ⬇️ death day 29 (p=0.07) @RheumNow #EULAR2021 Abstr#LB0001 https://t.co/VBPyv4Kbv7
Richard Conway RichardPAConway ( View Tweet)
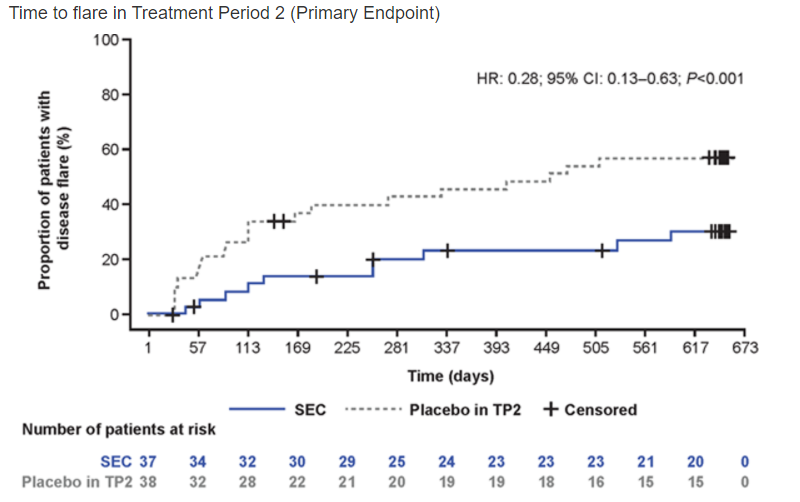
Dr Ruperto on secukinumab in ERA and JPsA subtypes of JIA. HR 0.28; 95%CI 0.13–0.63 p<0.001 for flare to week 100. 87.2%, 83.7%, 67.4%, 38.4% and 24.4% of pts achieved JIA ACR 30/50/70/90/100 @RheumNow #EULAR2021 Abstr#LB0004 https://t.co/envFupqqV2
Richard Conway RichardPAConway ( View Tweet)
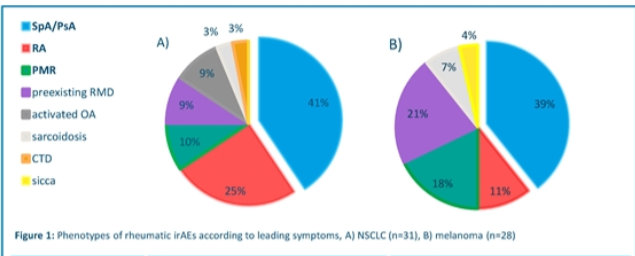
Dr Diekmann presents on rheumatic irAEs in different cancers. More common melanoma than NSCLC. SpA most common. Occurrence associated better tumour response. Good response to immunosuppression @RheumNow #EULAR2021 Abstr#POS0293 https://t.co/0tSHSbRq40
Richard Conway RichardPAConway ( View Tweet)
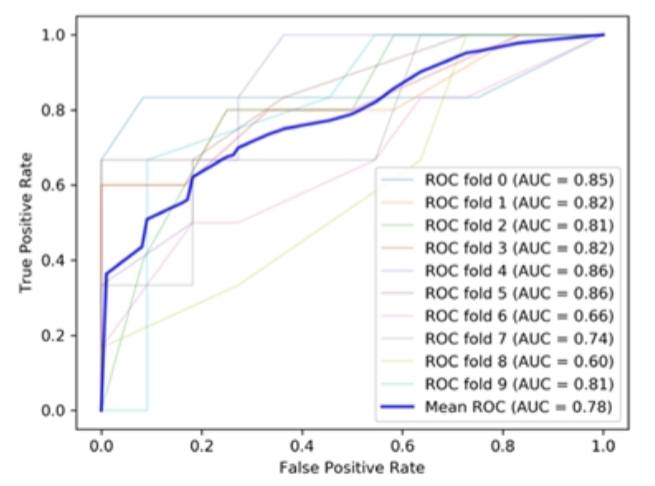
Dr Chatzis presents risk prediction model for MALT lymphoma in Sjogrens. Cryoglobulinaemia and ESSDAI at diagnosis only predictors. AUROC 0.78 for model @RheumNow #EULAR2021 Abstr#POS0290 https://t.co/cLmiMgnpwF
Richard Conway RichardPAConway ( View Tweet)

Quiz yourself.
Which of the following is NOT a risk factor for HCQ associated conduction abnormalities? #EULAR2021 @RheumNow
k dao KDAO2011 ( View Tweet)

Deep learning for SLE #POS0351
👉use of AI to ID potential targets/dz pathways
👉gene ranked using metric expression & interactivity
👉many IFN and JAK/STAT genes ranked high
👉strong assc w/new pathways related to CD4+Tcell different'n, macs, denditric cells #EULAR2021 @rheumnow https://t.co/JF7NFeX4E7
k dao KDAO2011 ( View Tweet)
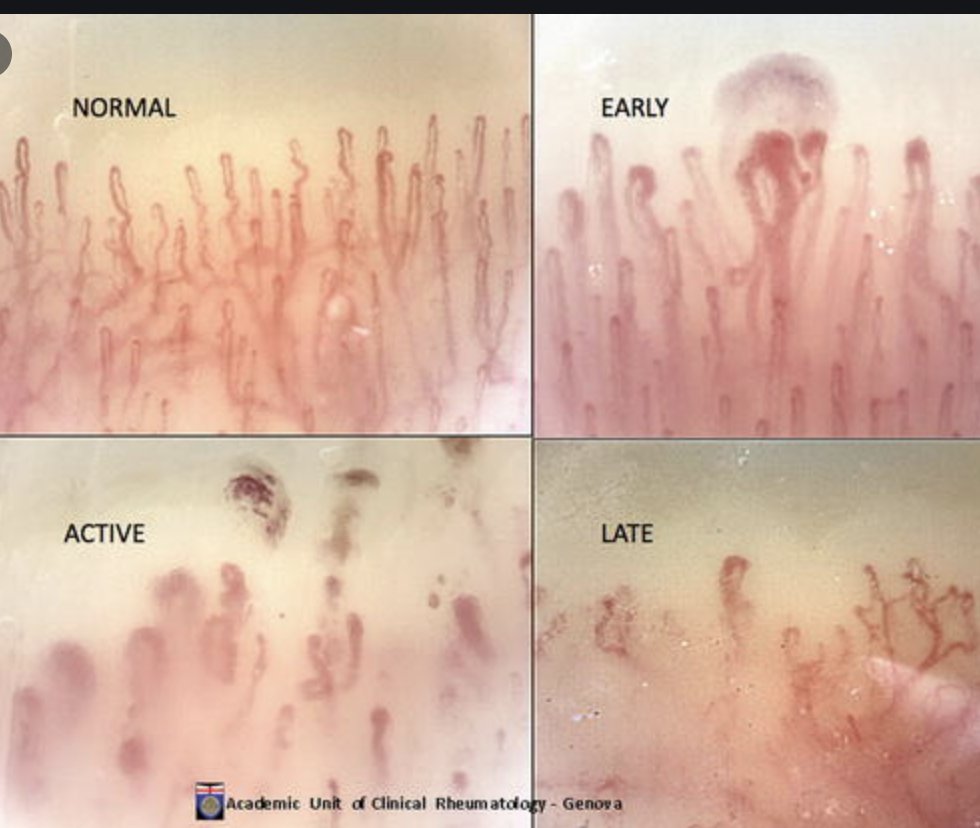
Not an expert in capillaroscopy? Not an issue! https://t.co/jHNKDtafQC: an automatic image reading system able to detect:
-Normal capillaries Se 79.8% Spe 82%
-Megacapillaries Se 89.4% Spe 78.8%
-Hemorrhages (Se 71% She 73.9%) compared to expert
#POS0256 #EULAR2021 @Rheumnow https://t.co/MR8enBO0de
Aurelie Najm AurelieRheumo ( View Tweet)
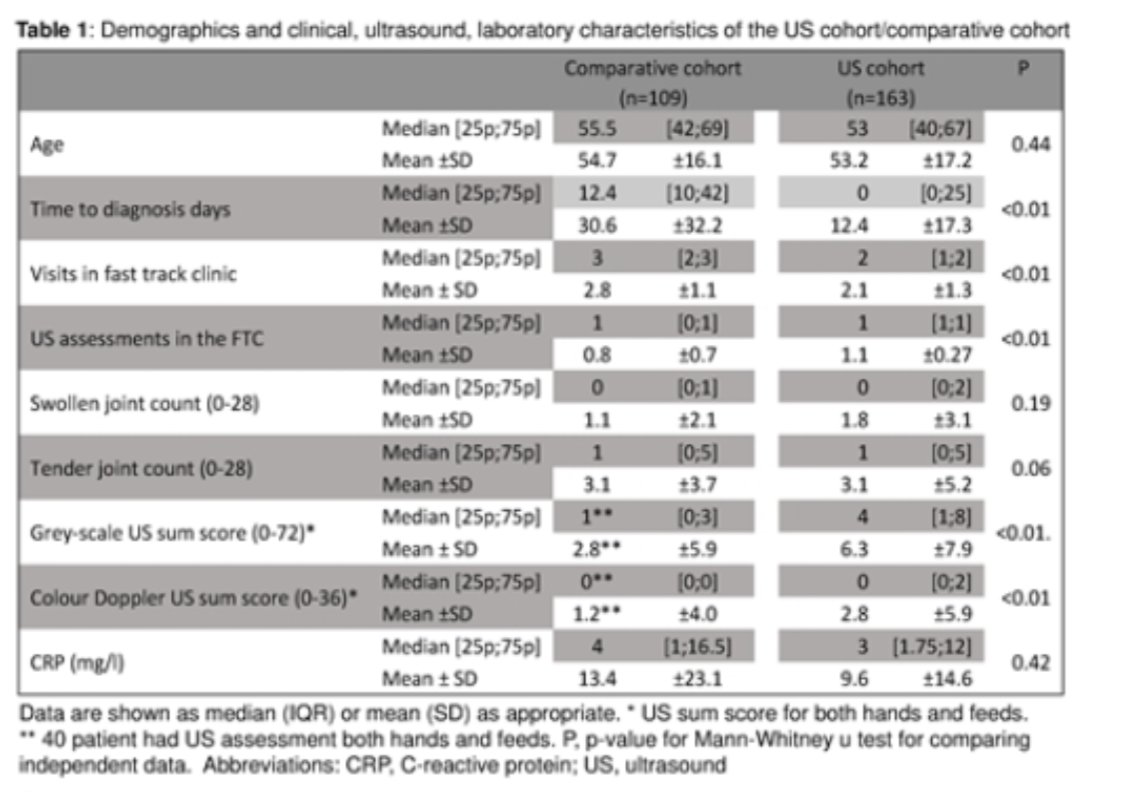
Wait, let me scan your joints first! Early arthritis clinics: US examination prior first clinical review in cohort of 163: ⬇️
⭐️time to diag from mean 31 days to 12
⭐️n° of clinical visits - 114 visits over a year
⭐️n° of revised diag at 1yr 5 vs 16% #POS0260
#EULAR2021 @Rheumnow https://t.co/CLkNqZUJVQ
Aurelie Najm AurelieRheumo ( View Tweet)
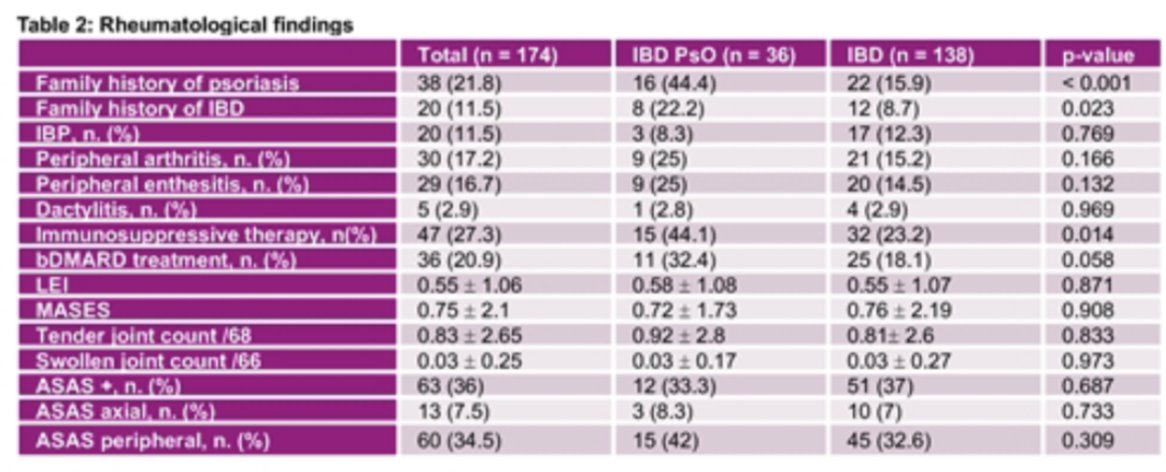
Subclinical US enthesitis in 174 IBD patients w/ or w/out PSO:
-High prevalence of structural damage ≥1 enthesis 83.4%
-≥1 active enthesitis 25%
in IBD + PSO:
-⬆️ ≥ 1 thickened enthesis 86% vs 64% p=0.009)
-⬆️of PD signal at knee examination.
#POS0265 @RheumNow #EULAR2021 https://t.co/8f7G5ku1kc
Aurelie Najm AurelieRheumo ( View Tweet)

Adelphi RA Disease Specific Program: Physician survey on T2T strategy implementation in RA. #POS0305
What is main objective in RA T2T?
@Rheumnow #EULAR2021
Aurelie Najm AurelieRheumo ( View Tweet)



