All News
Adverse Effects of Low-Dose Methotrexate from the CIRT Trial
Low-dose methotrexate (LD-MTX) was studied in high risk cardiac patients in the cardiac intervention trial (CIRT), but was prematurely ended for not showing a change in cardiovascular event rates. Nonetheless this trial studied the safety and adverse event rates of LD-MTX and those results are reported in the current issue of Annals of Internal Medicine.
Bimekizumab Effective in Active Psoriatic Arthritis
Interleukin 17 IIL-17) is important in the pathogenesis of psoriatic disease, with most current approaches targeting IL-17A. Now there is a noveal approach showing that dual neutralisation of IL-17A and IL-17F in psoriatic arthritis arthritis patients results in clinically significant improvement.
Read ArticleDMARD Inertia by Registry Rheumatologists
A registry study of metric use (primarily RAPID3 and CDAI) in the treatment of rheumatoid arthritis (RA) patients shows that, even in the face of moderate or high disease activity, treatment changes by rheumatologists were relatively low (35.6–54.6%).
Read ArticleUpadacitinib Effective in Phase 3 Psoriatic Arthritis Study
Abbvie has announced top line results of their SELECT-PSA trial of upadacitinib (UPA), wherein both the 15 and 30 mg doses met the primary endpoint of ACR20 response at week 12 and demonstrated radiographic inhibition at week 24.
Read ArticleWhich Biologics are Best in Psoriasis
A metanalysis of phase II, III and IV trials in moderate to severe plaque psoriasis suggests comparative efficacy biologic treatments, but that that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were shown to have the past skin (PASI) response rates.
Read ArticleLimited Advantage to Very Early vs. Delayed Etanercept in RA
The VEDERA study sought to confirm whether the very early introduction of first-line etanercept+methotrexate (ETN+MTX) was superior to treat-to-target MTX (MTX-TT) in patients with early RA.
Read ArticleBook Review: “Great Health Care Value: Chronic Diseases, Practice Teams and Population Management”
The US healthcare market has evolved into an incredibly expensive system that often does not deliver good medical outcomes. While most of us know these problems exist, we rarely have up-to-date data or can offer alternatives to the way we manage care, especially to the chronically ill who consume much of our health care dollars. In their book, authors Tim Harrington, MD and Andrew Johnson, MS, MBA offer insights, evidence and experience on how we may do our part to improve the management of chronic rheumatic/orthopedic issues.
2019 EULAR Recommendations for the Management of Rheumatoid Arthritis
The 2019 update to the EULAR recommendations on the use of synthetic and biological disease-modifying antirheumatic drugs in rheumatoid arthritis (RA) have been published in Annals of Rheumatic Disease - highlighting the efforts of an international consensus committee effort.
In the end, the task force put forth 5 overarching principles and 12 recommendations concerning use of conventional synthetic DMARDs, glucocorticoids, biological DMARDs, biosimilar DMARDs, and targeted synthetic DMARDs (the Janus kinase (JAK) inhibitors tofacitinib, baricitinib, filgotinib, upadacitinib).
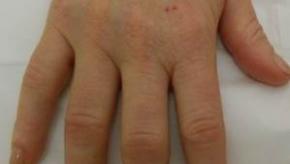
Low Risk of Inflammatory Arthritis in Hidradenitis Suppurativa Patients
JAMA Dermatology reports that after the onset and diagnosis of hidradenitis suppurativa (HS), such patients have a higher risk of certain forms inflammatory arthritis.
Read ArticleBiosimilars for Rheum Disease: Failure to Launch
The availability of biologic biosimilars has thus far had negligible impact on prescribing practices in the United States, in stark contrast to what has been observed in some European countries, researchers reported.
Read ArticleRheumNow Podcast- The Down Side of Steroids (1.17.20)
Dr. Jack Cush reviews the journal reports and news from RheumNow.com.
Be sure to register for RheumNow Live 3/13/2020 in Fort Worth
Rheumnow.live.
NSAID Safety Guidelines
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for acute or chronic arthritis, headache, visceral pain, postoperative pain, etc but come with a small but significant risk of serious adverse effects, including hypertension, cardiovascular disease, kidney injury and GI complica
Read ArticleTULIP2 - Anifrolumab Succeeds in Lupus
NEJM has published the results of the TULIP2 trial with anifrolumab, an alpha interferon blocker, in the treatment of systemic lupus erythematosus, showing significant improvement (over placebo) in multiple lupus outcome measures, including BICLA, SRI-4, CLASI and others.
Should We Screen Younger Postmenopausal Women for Osteoporosis?
Do postmenopausal women, under age 65 years, need a baseline BMD testing? A JAMA Insights review suggests that the absolute risk of fracture is low in this group and that USPSTF guidelines should be considered - that we should be screening women younger than 65 years who are at increased risk of osteoporosis and we should be using a formal risk assessment tool to identify candidates for bone density testing.
Read ArticleRheumatologists Ranked #1 in Happiness (Again)
Medscape has reported the results of its 2020 annual physician survey, This year rheumatologists (60%), general surgeons (60%), public health and preventive medicine physicians, and allergists/immunologists are the "happiest" outside of work compared to other specialists, according to Medscap
Read ArticleSteroids Up the Risk of Organ Damage in SLE
Lancet Rheumatology has reported the results of a multicenter follow-up study of systemic lupus erythematosus (SLE) patients showing that organ damange is linked to glucocorticoid use, independent of clinical or serological disease activity.
Read ArticleACR-Arthritis Foundation Treatment Guidelines for Osteoarthritis
Today, the American College of Rheumatology (ACR), in partnership with the Arthritis Foundation (AF), released the 2019 ACR/AF Guideline for the Management of Osteoarthritis of the Hand, Hip and Knee.
Read ArticleTaltz Shines in Non-Radiographic Axial Spondyloarthritis
Ixekizumab (IXE), an interleukin-17A (IL-17A) inhibitor, was recently approved for use in ankylosing spondylitis (also known as radiographic axial spondyloarthritis- axSpA).
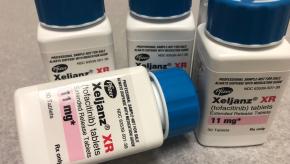
Read ArticleBest of 2019 - Is Methotrexate Necessary with Tofacitinib?
Rheumatoid arthritis patients taking tofacitinib (Xeljanz) plus methotrexate who achieved low disease activity (LDA) may be able to withdraw from the latter agent without significant worsening of disease activity, a researcher reported at EULAR 2019 in Madrid.
Read ArticleBest of 2019 - War on RA - Part 1: Walk on the Moon
It’s a great time to be a rheumatologist and to manage RA. But, if you keep doing what you’re doing, you’re going to keep getting what you’ve got.
Read Article