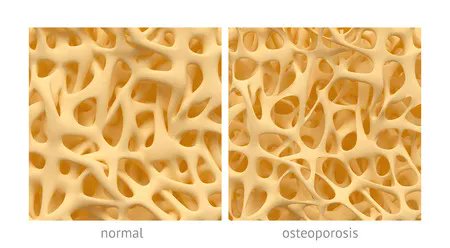
On Dec. 19, 2022, the U.S. Food & Drug Administration (FDA) approved subcutaneous abaloparatide (Tymlos) for the treatment of men with osteoporosis at high risk of fracture https://t.co/igwxVCdKuo https://t.co/COSmDrsv4p
Links:
15-02-2023



