All News
RheumNow Podcast – ACR 2020 Epilogue (11.20.20)
Dr. Jack Cush reviews a few more ACR 2020 abstracts and presents a new plan to learn or take in ACR20 content.
Read Article
Warfarin and End-Stage Osteoarthritis: Dr. David Liew ( @drdavidliew) #ACR20
https://t.co/hHZTL48DTI https://t.co/X4BDGBOGFq
Links:
Dr. John Cush RheumNow ( View Tweet)

#BestACR20Tweets
RT @MeralElRamahiMD : When do you think we are going to get a DMARD for OA? 50% or more over age 60 will have osteoarthritis, yet we are yet to discover a promising disease modifying therapy. #ACR20 @ElaineHusniMD
Dr. John Cush RheumNow ( View Tweet)
#BestACR20Tweets
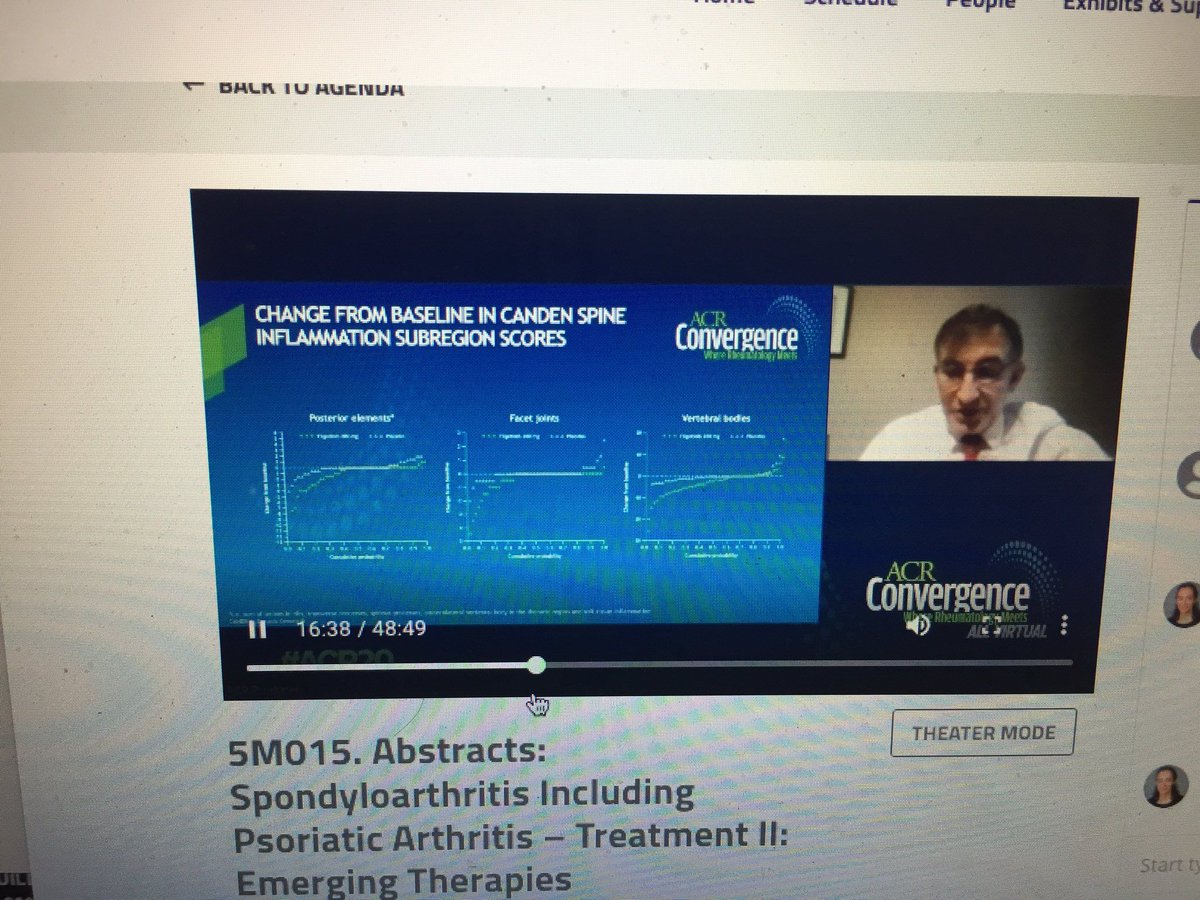
RT @Janetbirdope : Filgo decreased MRI inflammation of many structures early in Rx in SpA which seems novel says w Maksymowych 5M015 @RheumNow #ACR20 @CRASCRRheum #ACRbest https://t.co/5HQiIettjh https://t.co/NwFMkLIhWw
Links:
Dr. John Cush RheumNow ( View Tweet)

MAGIC Syndrome in Relapsing Polychondritis: Dr. Bella Mehta #ACR20
https://t.co/fjlB2T4o3E https://t.co/OBlccrMhpJ
Links:
Dr. John Cush RheumNow ( View Tweet)

Listen to our Psoriatic Arthritis Panel Discussion podcast with Drs. Artie Kavanaugh, Jack Cush, Eric Ruderman and Alexis Ogdie-Beatty. #ACR20 Listen via the link below or Apple/Android podcasts.
https://t.co/BbOyuM4kZV https://t.co/7Y1b6vmYy8
Links:
Dr. John Cush RheumNow ( View Tweet)

Check out our #ACR20 video. Dr. Kathryn Dao, ( @KDAO2011) breaks down the impact of exercise on sleep.
https://t.co/a9rtO3CVj3 https://t.co/aWVVGJ6Th1
Links:
Dr. John Cush RheumNow ( View Tweet)

XRay Bone Erosions in CCP+ Patients with Dr Eric Dein ( @ejdein1) #ACR20
https://t.co/f6RnoExBgk https://t.co/MJCp3sR1UZ
Links:
Dr. John Cush RheumNow ( View Tweet)

Check out of video by Dr. Rachel Tate ( @uptoTate ) titled "Treat to Target in Spondyloarthritis". #ACR20
https://t.co/7ThVtmguFI https://t.co/on9m0qtse3
Links:
Dr. John Cush RheumNow ( View Tweet)

Check out this video on combo therapy v monotherapy (SEAM-RA Trial) with Dr. Richard Conway (@RichardPAConway). #ACR20
https://t.co/iNsmKHSnMq https://t.co/tELmueVEK0
Links:
Dr. John Cush RheumNow ( View Tweet)

Watch our #ACR20 video recap titled "Treatment of Dermatomyositis Studies" with Dr Robert Chao (@doctorRBC). Dr. Chao reviews two important abstracts - #0955 and #0958 - on the treatment of dermatomyositis as presented at the 2020 ACR annual meeting.
https://t.co/1hiTZQVjlL https://t.co/CW9OGHTuJg
Links:
Dr. John Cush RheumNow ( View Tweet)

#BestACRTweets20
RT @RHEUMarampa : IgG4-RD tx: GCs are mainstay tx starting at 30-40mg/d tapered gradually but 30% of pts will have recurrences
Potential therapeutic targets to ⬇GC toxicity
✏B cell/plasmablasts (promising)
✏T cells (anecdotal) #ACR20 https://t.co/htu849kng3
Links:
Dr. John Cush RheumNow ( View Tweet)

#BestACR20Tweets
RT @synovialjoints : New mode of action treatment in AS. The SELECT-AXIS 1 showed Upadacitinib 15mg od showed sustained efficacy over 1 year @RheumNow #ACR20 Abstr#2023 #ACRbest https://t.co/Auo3AMNTDw
Links:
Dr. John Cush RheumNow ( View Tweet)

What is the SEAMless transition from combination Etanercept to Methotrexate? Dr. Jonathan Kay takes a deep dive into plenary abstract #0939 presented at #ACR20 annual meeting, reviewing two previous clinical trials that also looked at this question.
https://t.co/58raeaE8Mr https://t.co/ZP2P0IZ1Fc
Links:
Dr. John Cush RheumNow ( View Tweet)

View one of our fantastic #ACR20 video titled "Staying on Therapy in RA" with Dr. Janet Pope ( @Janetbirdope)
https://t.co/imCpxVs2N8 https://t.co/AcTBGaapig
Links:
Dr. John Cush RheumNow ( View Tweet)

#BestARC20Tweets
"RT @drdavidliew : Great to sit down with @LCalabreseDO @Sarah_L_Mackie @RichardPAConway a few hours ago and chat about two very interesting GCA therapeutics abstracts: https://t.co/V3fzB0W3Ko
great discussion!
#ACR20 @RheumNow"
Links:
Dr. John Cush RheumNow ( View Tweet)
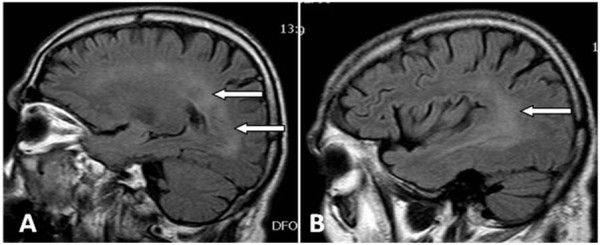
Read our article titled: Best Imaging Techniques for non ANCA Vasculitis by Dr. Alexa Meara ( @lexmeara) as discussed at #ACR20 https://t.co/CyfC8bdq42 https://t.co/Ay7Sw0eMTY
Links:
Dr. John Cush RheumNow ( View Tweet)

What was your favorite abstract or study presented at #ACR20? We've rounded up our top choices. #ACRbest https://t.co/50hpUUSCBD https://t.co/09t2kziLQr
Links:
Dr. John Cush RheumNow ( View Tweet)

What is the SEAMless transition from combination Etanercept to Methotrexate? with Dr. Jonathan Kay #ACR20
https://t.co/58raeaE8Mr https://t.co/FUIidcoy4I
Links:
Dr. John Cush RheumNow ( View Tweet)
The #ACR20 convergence highlighted a shift in gout management and uric acid lowering therapy (ULT). Read more about mitigating immunogenicity when using uricase therapies as ULT in an article by Dr. Alexa Meara ( @lexmeara) https://t.co/5AfyFhJGqp https://t.co/Sv1BiI0Uwd
Links:
Dr. John Cush RheumNow ( View Tweet)




