All News
IMPACT Study - Certolizumab Efficacy in APS Pregnancies
A pilot trial assessed the safety and value of tumour necrosis factor α inhibitor treatment with certolizumab in patients with high-risk pregnancies with antiphospholipid syndrome (APS).
Lancet viewpoint on early imaging in axial SpA. Over-reliance on BM edema = incr in misDx. Newer tech (high-res sequences, quantitative MRI, radiomics, artificial intelligence) may improve Dx precision. Remember the SI is susceptible to mechanical stress https://t.co/E80GyQGIM0 https://t.co/csQrBPHAlM
Dr. John Cush RheumNow ( View Tweet)

JAMA CME review of Immune Thrombotic Thrombocytopenic Purpura (iTTP) - thrombotic microangiopathy w/ hemolytic anemia (MAHA) & thrombocytopenia. iTTP incidence is 2-6 per million. Caused by Abs to ADAMTS13), w/ low ADAMTS13 activity vWF multimers bind platelets https://t.co/kwKXUciBd1
Dr. John Cush RheumNow ( View Tweet)

Annals of Internal Medicine has published a CME review of LYME DISEASE. Most Lyme is Dx by onset of the erythema migrans rashes,. Lyme Ab testing is of low sensitivity at onset but more sensitive w/ time & CNS, CV complications. or Mono- or oligoarticular arthritis https://t.co/NfL0dabeGe
Dr. John Cush RheumNow ( View Tweet)

“Courage is what it takes to stand up and speak; courage is also what it takes to sit down and listen.”
– Winston Churchill https://t.co/5YMQppiMSG
Dr. John Cush RheumNow ( View Tweet)
Hydroxychloroquine for Everyone
Nearly 25 years ago, while lecturing on best therapies for rheumatoid arthritis (RA), I loudly stated that hydroxychloroquine was “useless” and, deservedly, I was “boo-ed” off stage. My point then was that rheumatologists needed to be aggressive, https://t.co/qKnObzr5ib
Dr. John Cush RheumNow ( View Tweet)

Biologics in Pregnancy Patients With Autoimmune Disease
A large cohort, claims data study shows that among pregnant women receiving biologic therapies for autoimmune conditions, 72% continued their biologics pregnancy, more so among inflammatory bowel disease (IBD) patients https://t.co/CH60gShlH0
Dr. John Cush RheumNow ( View Tweet)

Lupus Clinical Slides: Set 1
https://t.co/zFQqHfl8Ch https://t.co/kvVNAS17ec
Dr. John Cush RheumNow ( View Tweet)

SLE, Antiphospholipid Antibodies, and the Placenta
It is well established that women with SLE are at increased risk for adverse pregnancy outcomes, including preeclampsia, preterm birth, and pregnancy loss. However, the pathophysiologic mechanisms driving these complications https://t.co/z0BOT3xsuQ
Dr. John Cush RheumNow ( View Tweet)
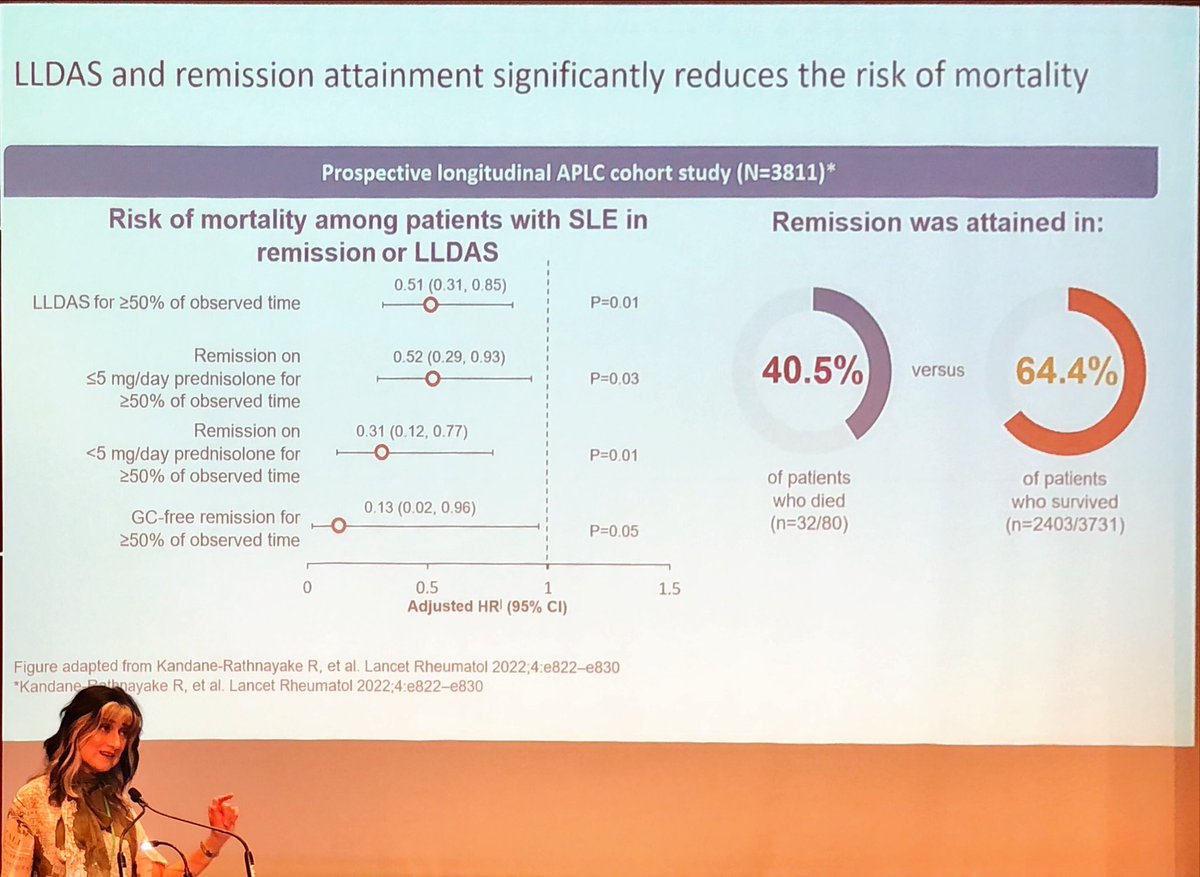
Why DORIS or LLDAS #remission
🤔
⬇️mortality
⬇️damage
⬆️QoL
Dr Nikpour at On Rheum Assoc
Shows Remission > LLDAS
no pred ✅ best
@RheumNow https://t.co/tFcPrkd5Vd
Links:
Janet Pope Janetbirdope ( View Tweet)

🍋 or ‘Lyme’
- is it Lyme disease?
Dr Elizabeth Stringer discusses Eastern Canada 🇨🇦
Epidemic of #Lyme #arthritis
Which mimics #pauciarticular #JIA
When it is #chronic
Work up of all oligoarthritis in endemic areas in kids
Includes
Validated Lyme test
@ORAexec #ORA25 https://t.co/WxU1vaOa7z
Janet Pope Janetbirdope ( View Tweet)
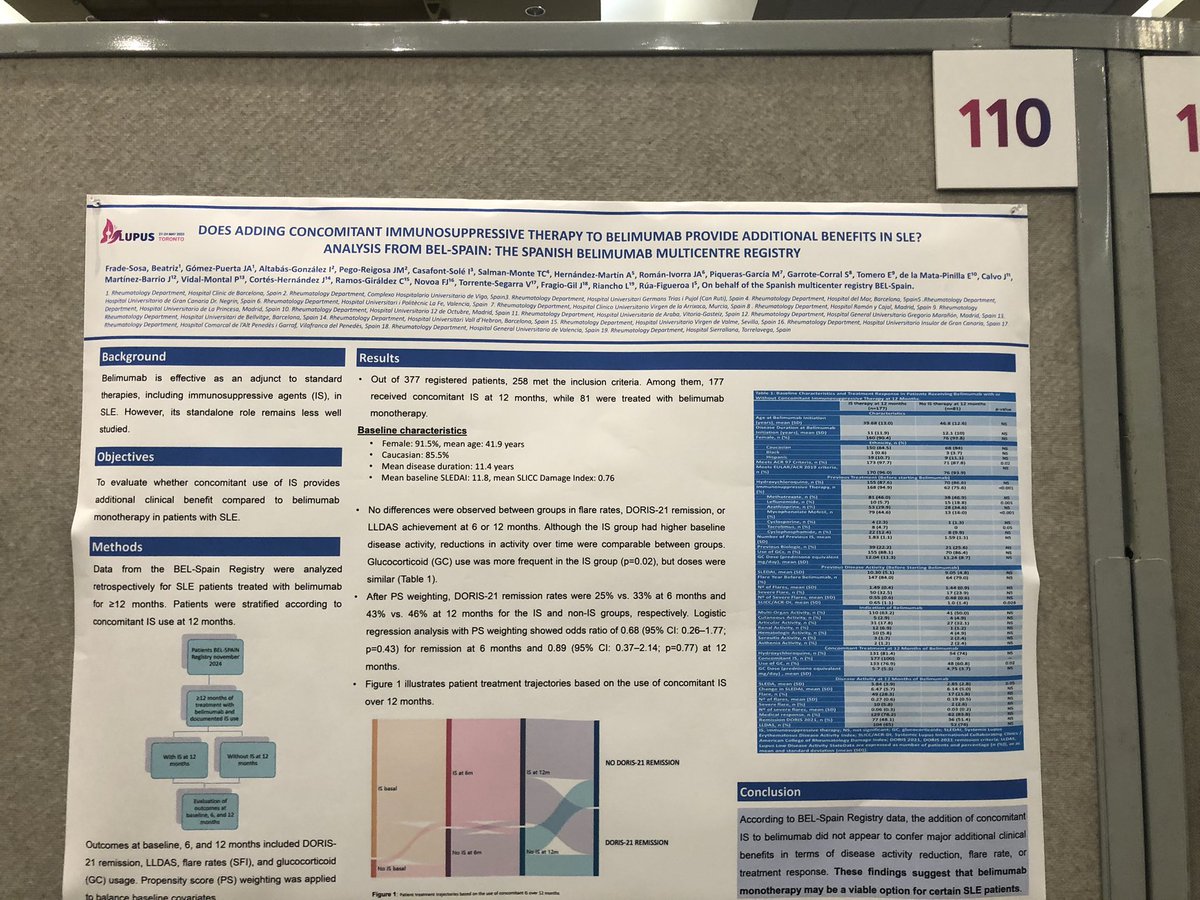
Does ADDING an #immunesuppressive
To
#belimumab in #SLE give more benefit?
❎
NO
#RWE from Spain suggests no added improvement adding IS to Belimumab
Should you switch Rx then? 🤷♀️
@RheumNow #LUPUS2025 poster110
#ClinicalPearl https://t.co/C3ydArMx7x
Links:
Janet Pope Janetbirdope ( View Tweet)

Treat to target 🎯 #SLE #recommendations
Considerations are nuanced and Impt
Lowest clinical disease activity is goal
Try for remission
🎯 Get patients off #glucocorticoids
If not ⬇️low dose #prednisone
@RheumNow #LUPUS2025 https://t.co/HlBeh2FLxP
Links:
Janet Pope Janetbirdope ( View Tweet)

Lupus QD Clinic - Rhupus
dsDNA+ SLE Plus CCP+ RA = Rhupus
https://t.co/lyV7fgd1vp https://t.co/Ptw0dmyqCj
Dr. John Cush RheumNow ( View Tweet)

Lupus in an empty house
The full house immunofluorescence pattern is the classic histopathologic finding of lupus and lupus nephritis. Glomerular deposits staining for IgG, IgA, IgM, C3 and C1q can help confirm a suspected diagnosis of systemic lupus erythematosus (SLE). This https://t.co/Zcywc5r9q9
Dr. John Cush RheumNow ( View Tweet)

Lupus Unlocked: Pregnancy & SLE
📅 May 27, 2025
6:00 PM CT | 7:00 PM ET | 4PM PT
Join us for our final Lupus Unlocked webinar this month on pregnancy and SLE. In this webinar, panelists will discuss issues such as congenital heart block, lupus nephritis treatment, teratogenic https://t.co/gouTN7uzCo
Dr. John Cush RheumNow ( View Tweet)

Unmet needs in #lupus are many
👇
Timely #diagnosis
Morbidity - CV, metabolic, OP, mental health
Premature mortality - CAD, active #SLE, infection
Issues w pregnancy
#steroid side effects
⬇️QoL
With partnerships we can ✅
Pts
HCPs
researchers
Pharma
Gov’t
#LUPUS2025
@RheumNow https://t.co/9T9rIrM1hV
Janet Pope Janetbirdope ( View Tweet)
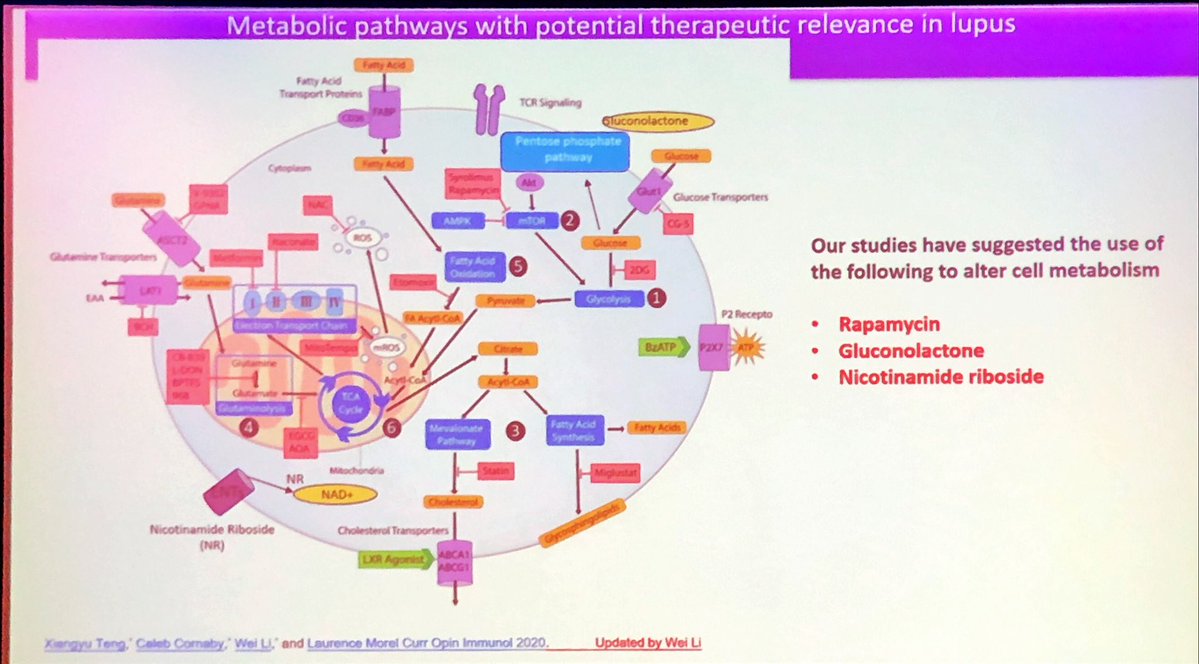
IL2 low dose in #SLE
seems to + alter immune regulation
Multiple targets to explore in #lupus
Unravelling pathophysiology in SLE
CD40L
TLR7,8
#rapamycin type drugs
CAR-T w novel CARS
Etc
#LUPUS2025
@RheumNow https://t.co/jeycgg4ZhT
Links:
Janet Pope Janetbirdope ( View Tweet)

>200 genes 🧬 implicated in #SLE
Complex indeed!
And
▶️ 7 clusters of signalling pathways
So we have to have many Rx options
Ab patterns vary
LN flares can have v diff pathways between Pts
#LUPUS2025 @RheumNow
Unravelling #LUPUS2026 https://t.co/LEKN363194
Links:
Janet Pope Janetbirdope ( View Tweet)

We are not (yet) in #SLE
Getting
👇
Correct drug
In right pt
At right time
At correct individual dose
Even more problematic is in a renal bx - 2 glomeruli beside each other have v different cytokine expressions
Unravelling #LUPUS2025
@RheumNow https://t.co/yGBvhFkPKY
Links:
Janet Pope Janetbirdope ( View Tweet)




