All News
Diagnosing Neuropsychiatric SLE (5.23.2025)
Dr. Jack Cush reviews the news and journal reports from RheumNow.com - including views on the vagus nerve, NPSLE and CAR-T mania.
Read Article
Here’s who flares in #SLE
least to most
Persistently neg antiDNA
Persistently + antiDNA
conversion of neg to + antiDNA
So are we over ordering antibodies in 1st 2 groups 🤷♀️
Unravelling #LUPUS2025
@RheumNow https://t.co/1Feau7f199
Janet Pope Janetbirdope ( View Tweet)
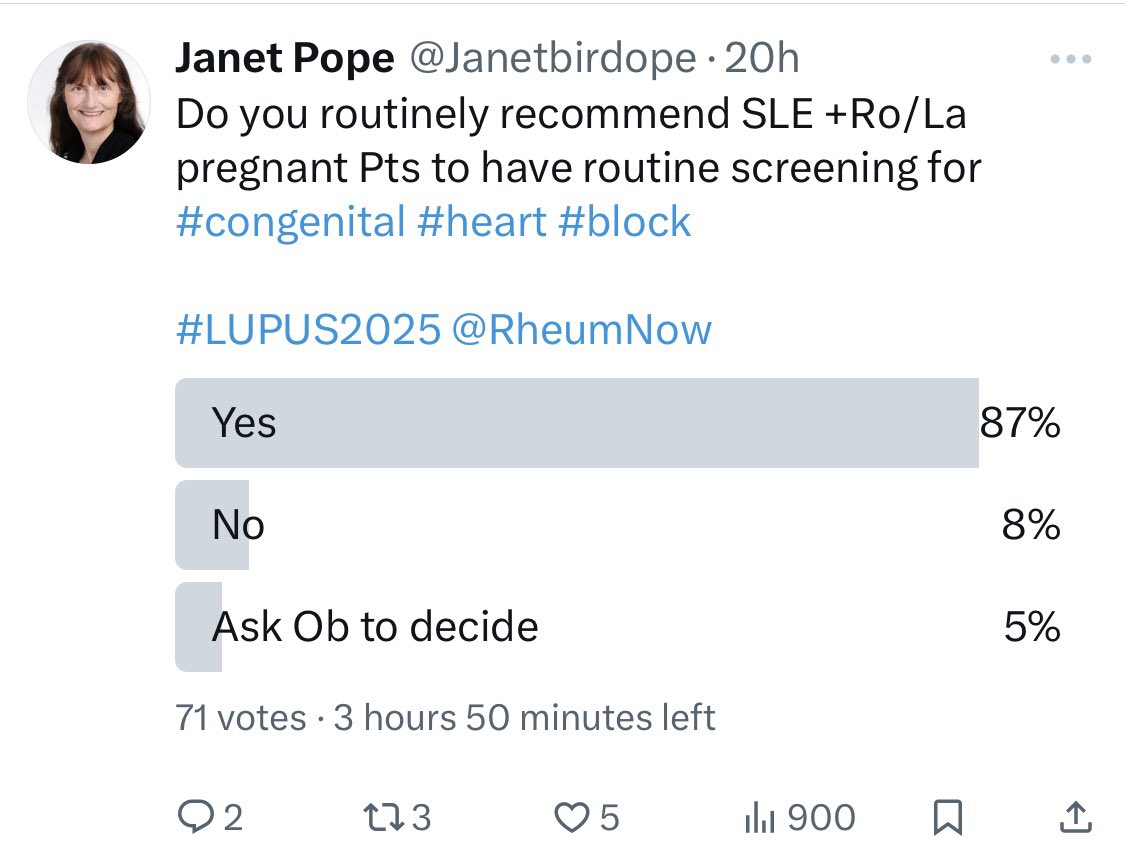
controversy re
👇
Monitoring for +Ro pregnant re
#congenital #heart #block
87% Y
echo is possibly too late
No ideas if flourinated #steroids work
But many thing we should do something
Guidelines differ on recommendations
Would fetal home HR be better
#LUPUS2025 @RheumNow https://t.co/MY0oPjLnGs
Janet Pope Janetbirdope ( View Tweet)

An excellent presentation from Dr Pankti Mehta @PanktiMehta24
Re #Jaccoud’s
With
#IFN signature
if #erosive IA - often + CCP / RF
Vs
nonerosive often +RNP
@RheumNow #LUPUS2025 https://t.co/y6z4KbhCdy
Janet Pope Janetbirdope ( View Tweet)

The ANA pattern I hate
#DFS
Why
Dense fine speckled should R/O #SLE
But mimicking patterns that MAY Have #lupus
AC-2 pattern mimickers ex AC-4
Machine learning models to help
▶️improved accuracy
Fast saving hrs
#Artificial #intelligence
May Choi #LUPUS2025 @RheumNow https://t.co/9tk80ZOAWg
Janet Pope Janetbirdope ( View Tweet)
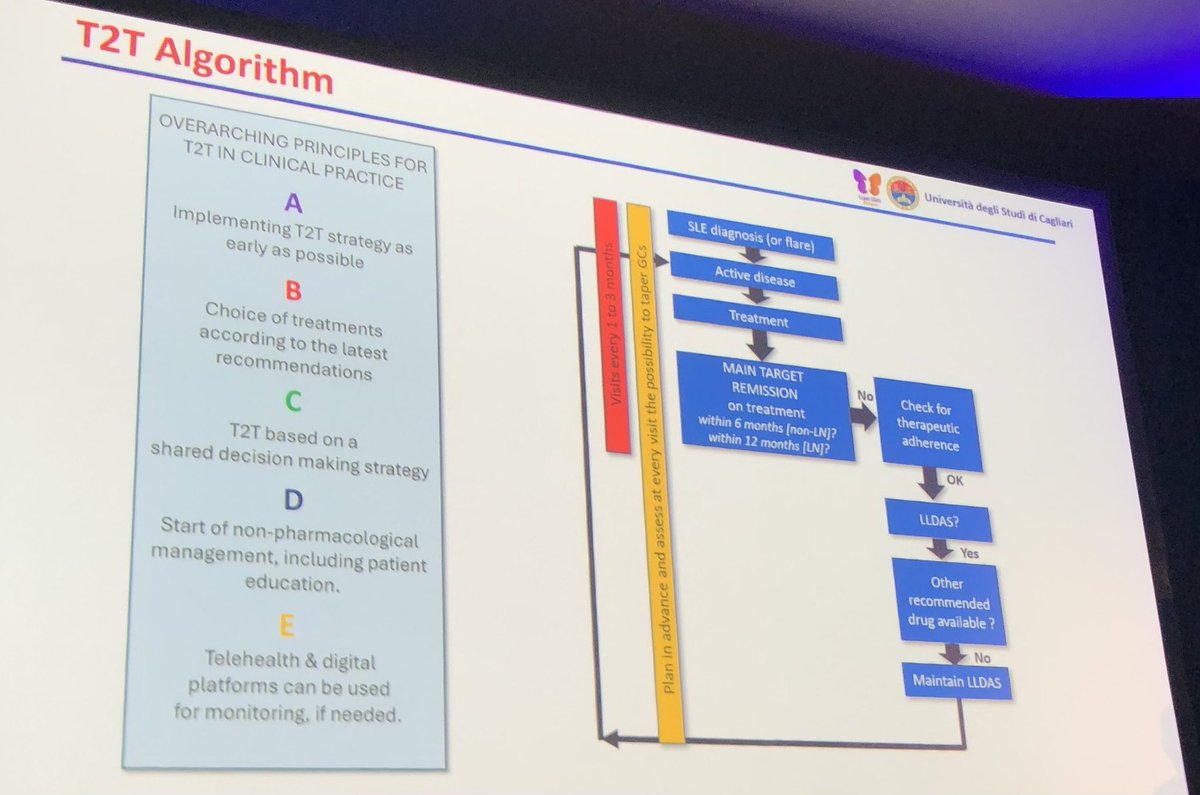
T2T in #SLE
Lots of #rheumatologists do NOT do a validated measure so #DORIS remission may be over estimated
But great algorithm for #lupus #Rx
@RheumNow
#LUPUS2025 https://t.co/mGoopRtK2F
Janet Pope Janetbirdope ( View Tweet)

Monitoring for #HCQ #retinal #toxicity
2 tests beyond visual fields are better than 1
OCT
Etc
But suggestion if low risk to do only every 5 yrs
I don’t agree as risk ⬆️ w disease duration
We do
Annual fields
and either
OCT or retinal photography
#LUPUS2025
@RheumNow https://t.co/TdPJ47jYEL
Links:
Janet Pope Janetbirdope ( View Tweet)

Subclinical #arthritis in #SLE
Do we look for it?
Should we care?
Certainly seems to exist on #MRI hands
More changes of course if tender joints
Not sure of clinical relevance
Optical imaging and POCUS was compared
#LUPUS2025 @RheumNow
ANCA Askanase presents
Janet Pope Janetbirdope ( View Tweet)

No Cancer Recurrence with Biologic DMARDs in RA
A prospective Danish rheumatoid arthritis (RA) registry study look at the risk of recurrence when a biological disease-modifying antirheumatic drugs (bDMARDs) was ued in patients with a a prior solid cancer (in remission) and https://t.co/T7G96cAlE6
Dr. John Cush RheumNow ( View Tweet)
Tocilizumab vs. Methotrexate in Rheumatoid Arthritis
A large randomized rheumatoid arthritis (RA) clinical trial compared subcutaneous tocilizumab (TCZ) vs oral methotrexate (MTX) vs. the combination of subcutaneous TCZ and MTX, and showed that TCZ was superior to MTX, either https://t.co/AS8MJaujIE
Dr. John Cush RheumNow ( View Tweet)

Why follow antiDNA and complements in
#lupus #nephritis #LN
#LUPUS2025 panel said
To detect flare
?to consider after 3 to 5 yrs of remission to taper Rx
?but not for early response to Rx
Just info from the panel with my opinion salted in
@RheumNow https://t.co/bFUfN0PsFF
Links:
Janet Pope Janetbirdope ( View Tweet)

deep thoughts in #lupus
Is ‘better’ the enemy of #remission?
Is a complete renal response <50% at the end of contemporary #triple #therapy
Something to celebrate?
▶️Yes better than old #SoC but a bit sobering
We need RCTs that radically change paradigm
#LUPUS2025 @RheumNow
Janet Pope Janetbirdope ( View Tweet)

Who with +ANA will become #SLE
Mostly similar cytokine signature
But ⬆️IFN and
more innate immune changes
👇
In those who developed #lupus
? prediction & Prevention model in +#ANA people 🤷♀️
yr in review E Vital’s paper
#LUPUS2025 @RheumNow @ZahiTouma https://t.co/khpqRlH8Nk
Janet Pope Janetbirdope ( View Tweet)
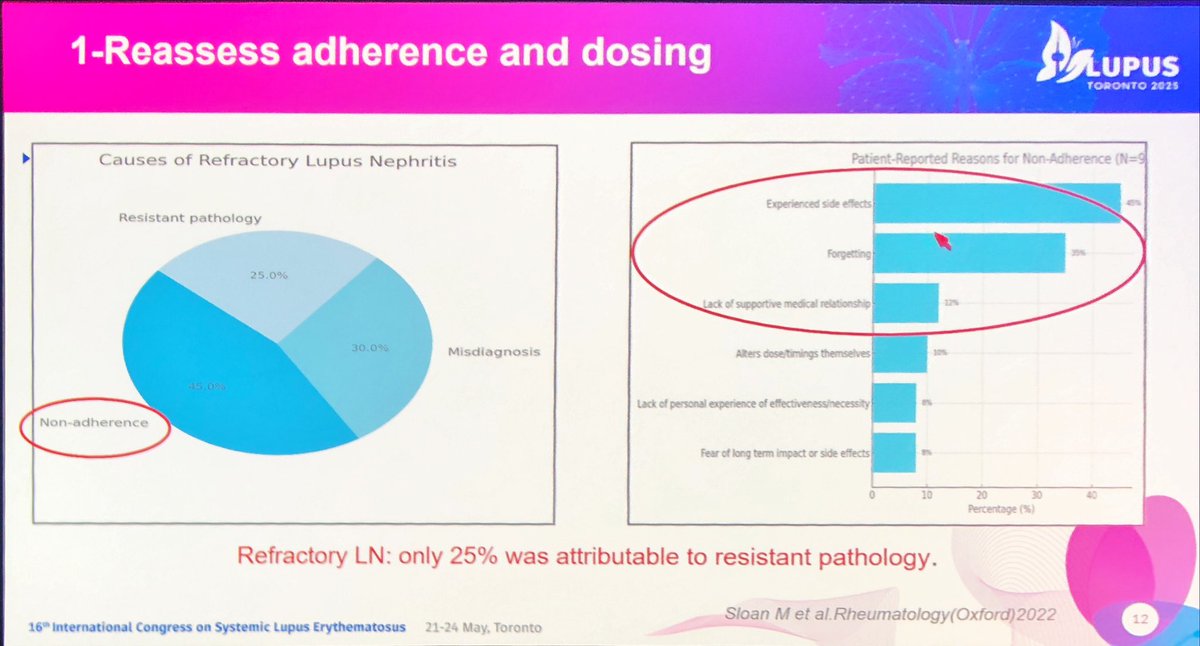
‘Oranges & green vegetables’
⬆️
#diarrhea in #MMF said Ana Malvar
bad #LN outcomes if #nonadherent
Good relationship with#HCP helps
#adherence
✅education to help deal with side effects
pts need to know
Why this Rx
?how long
S|E Rx
#ClinicalPearl
#LUPUS2025
@RheumNow https://t.co/RnFGwJSl1F
Janet Pope Janetbirdope ( View Tweet)
Who does worse with #SLE
#nature vs #nurture
South American #lupus #registries
⬆️ activity
⬆️damage
⬇️age
⬇️SES
⬆️SLE #antibodies
#Gladel registry and others vs Europe
Environment >genetics 🤔
#LUPUS2025 #45poster @RheumNow
@gponsestel https://t.co/XjiXHAMuGm
Links:
Janet Pope Janetbirdope ( View Tweet)

do’s & don’t #Lupus #nephritis
❎
Don’t sequential monotherapy
▶️ use #MMF + #biologic or #CNI
✅
Do a #biopsy
➡️ need to know
#dx
#px
Do target LOW proteinuria
Do treat comorbidities
Lipids
HTN
Fluids
BMI
?mood
No #NSAIDs
Do limit #pred
#SGLT2i
@RheumNow #LUPUS2025 https://t.co/D0vHQXYTvZ
Links:
Janet Pope Janetbirdope ( View Tweet)

Impt predictors of
#neuropsychiatric #SLE
#LUPUS2025 @RheumNow
No major predictors
But if explanation of other conditions ex
Past #cvA
#ischemic events
Doesn’t have to have
➡️Active systemic ##Lupus
➡️ ?ribosomalP
+Abs described in #CNS lupus
A real headache! https://t.co/iuWfB454EN
Janet Pope Janetbirdope ( View Tweet)

‘It’s #Lupus, it’s all in your head!’
➡️ Some patients with #SLE have heard this from others
At #LUPUS2025 in a packed session on #CNS lupus
#Dx is tricky
40% of #neuropsychiatric lupus have NEGATIVE #brain #MRI
👇r/o
infarct/bleed
Infection
Rx #steroids
@RheumNow https://t.co/yU1olg0uZU
Links:
Janet Pope Janetbirdope ( View Tweet)

Steroids prevention of #congenital #heart #block in +Ro
Mom
#DM #HTN #wt⬆️
Fetus
?no diff #cardiomyopathy
?learning &behavioural changes
Debate re screen #CHB
Most CHB are kids w CHB as mom is unknown to be +Ro
Angst w screening
High titre Ro Impt
@RheumNow #LUPUS2025
Janet Pope Janetbirdope ( View Tweet)





