All News
More Women with Autoimmune Diseases Die from Cardiovascular Disease
d
EurekAlert!
Women with the autoimmune diseases rheumatoid arthritis, lupus or systemic sclerosis may have a higher rate of death related to cardiovascular disease than men with the autoimmune diseases, according to new research published in the American Heart Association’s journal Circulation: Cardiovascular Quality and Outcomes.
Read ArticleD-Lay Trial: High-Dose Vitamin D Retards Multiple Sclerosis
A randomized clinical trial with oral high-dose cholecalciferol ( vitamin D3) was shown to prevent or delay the onset of clinically isolated syndrome (CIS), typical for multiple sclerosis (MS).
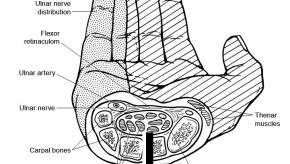
Read ArticleCarpal Tunnel Syndrome as a Harbinger of Rheumatoid Arthritis
d
MedPage Today
Rates of carpal tunnel syndrome were significantly greater in patients later diagnosed with rheumatoid arthritis, according to a large, long-running observational study.
Read Article
Using 16S rRNA gene amplification, Gut bacterial taxa from 53 CRPS pts compared to 52 controls. Differences were seen in microbiome and plasma short-chain fatty acid levels between CRPS patients and controls, w/ >90% accuracy https://t.co/TO7CCmmtD5 https://t.co/Gn9loJPNfM
Dr. John Cush RheumNow ( View Tweet)
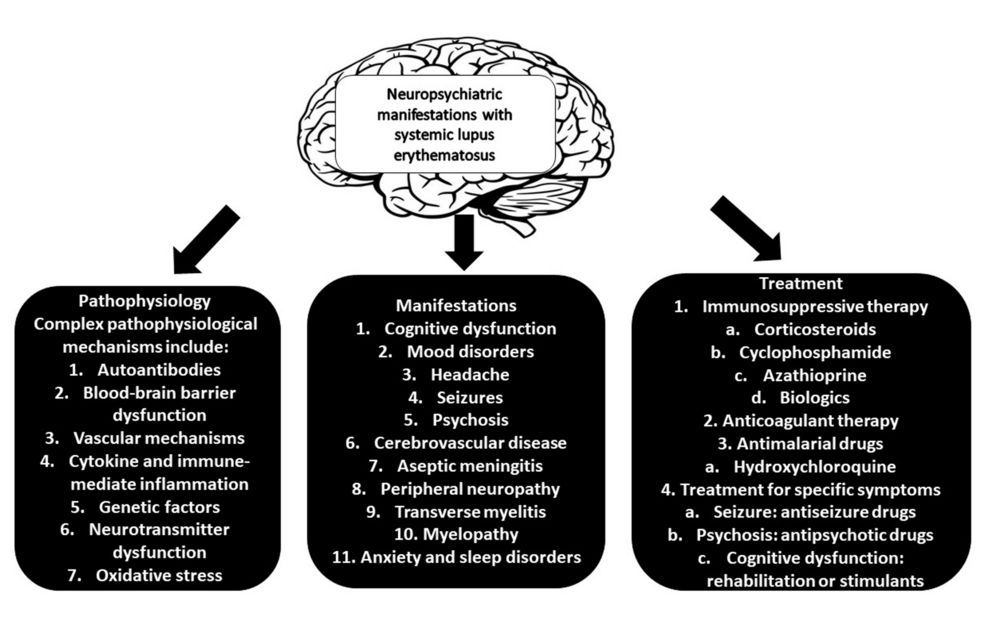
Full read review of Neuropsychiatric SLE. NPSLE criteria includes 12 central & 7 peripheral findings (psych, cognitive, Sz, CVA/TIA, neuropathy, MS-like). NPSLE AutoAbs are many: Abs against APL, LAC, RP, NMDA, NMO/AQP4, EC, SBSN, UCH-L1, TP1, GAPDH, MAP2, U1RNP https://t.co/1m7SXMA68Z
Dr. John Cush RheumNow ( View Tweet)
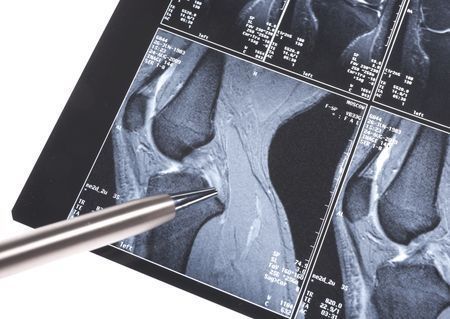
Finish Birth Cohort followed since 1986, who were asymptomatic, found abnormal MRI knee findings, esp cartilage defects in the patellofemoral (56%) & tibiofemoral joints (25%) joints. Small/doubtful patellofemoral (52%) & tibiofemoral (17%) osteophytes seen. Most w/ High BMI. https://t.co/nQKREE4XfU
Dr. John Cush RheumNow ( View Tweet)

GLP-1 agonists effective in Rxing MASH - metabolic dysfunction-assoc steatohepatitis (AKA NAFLD, NASH). DBRPCT w/ 1100 pts (mean BMI 34-35). A good perspective article from Sensible Medicine. https://t.co/NeaX5qcnPA https://t.co/5DHlKfH105 https://t.co/sCfvcm7g54
Links:
Dr. John Cush RheumNow ( View Tweet)
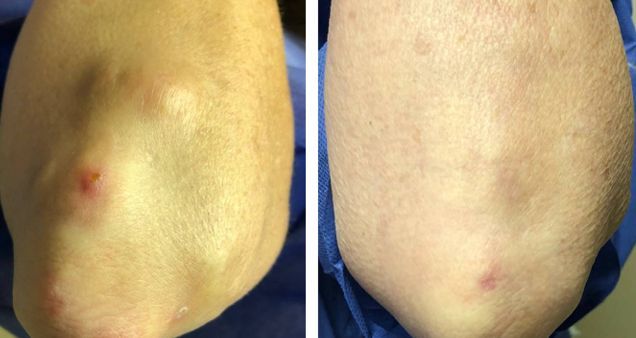
RA nodules respond to JAKi. Small case series of 7 established/refractory moderate-to-severe#RA pts w/ rheumatoid nodules who were treated w/ JAKi (tofacitinib, upadacitinib)-- 5/7 had complete resolution & 1 reduced nodules size (w/in 3-12 mos) on JAKi therapy. https://t.co/YGK1EGiJiJ
Dr. John Cush RheumNow ( View Tweet)

Prophylaxis Against PJP in SLE: I'll Pass with @EBRheum
https://t.co/tUGwziEYCg https://t.co/VqTa9jG8oj
Dr. John Cush RheumNow ( View Tweet)

Congrats to Dr. Virginia Pascual who was elected to American Academy of Arts and Sciences. Virginia isthe director of Gale & Ira Drukier Institute for Children’s Health & Ronay Menschel Professor of Pediatrics @ Weill Cornell Medicine. Her translational/basic research have been https://t.co/hOYi59xMC5
Dr. John Cush RheumNow ( View Tweet)

Stable SLE - Should you Withdraw Immunosuppressant or Glucocorticoids?
An open-label, single-centre, randomized controlled trial tested whether immunosuppressant (IS) withdrawal is noninferior to glucocorticoid (GC) withdrawal in SLE patients and found that IS withdrawal is https://t.co/T8Fu83GScH
Dr. John Cush RheumNow ( View Tweet)

Glucocorticoids in SLE: how to start, how to follow, how to stop
More than 70 years after their first use in rheumatology by Philip Hench, glucocorticoids (GCs) continue to be one of the main weapons to fight systemic lupus erythematosus (SLE). No other available medication https://t.co/H6N0N3BhhU
Dr. John Cush RheumNow ( View Tweet)
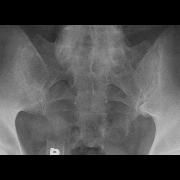
Pitfalls in sacroiliitis imaging: Bone marrow edema may also be seen in young-middle-aged postpartum women, & athletes & kids (ongoing bone growth) & w/ advancing age (DJD) https://t.co/bwS047wlmI https://t.co/FvWlEcZ5JP
Dr. John Cush RheumNow ( View Tweet)
single-centre study of 469 consecutive RA pts - 15 had VTE (3%). Strongest risk factor of VTE was the history of previous VTE (OR 44.7), recent hospitalisation (OR 6.82), diabetes (OR 11.23), and JAKi (OR 5.54) (Other studies show inflammation incr VTE risk) https://t.co/pBJDhXOgut
Dr. John Cush RheumNow ( View Tweet)
Using 16S rRNA gene amplification, Gut bacterial taxa from 53 CRPS pts compared to 52 controls. Differences were seen in microbiome and plasma short-chain fatty acid levels between CRPS patients and controls, w/ >90% accuracy https://t.co/TO7CCmmtD5 https://t.co/zM1wKxjGBX
Dr. John Cush RheumNow ( View Tweet)

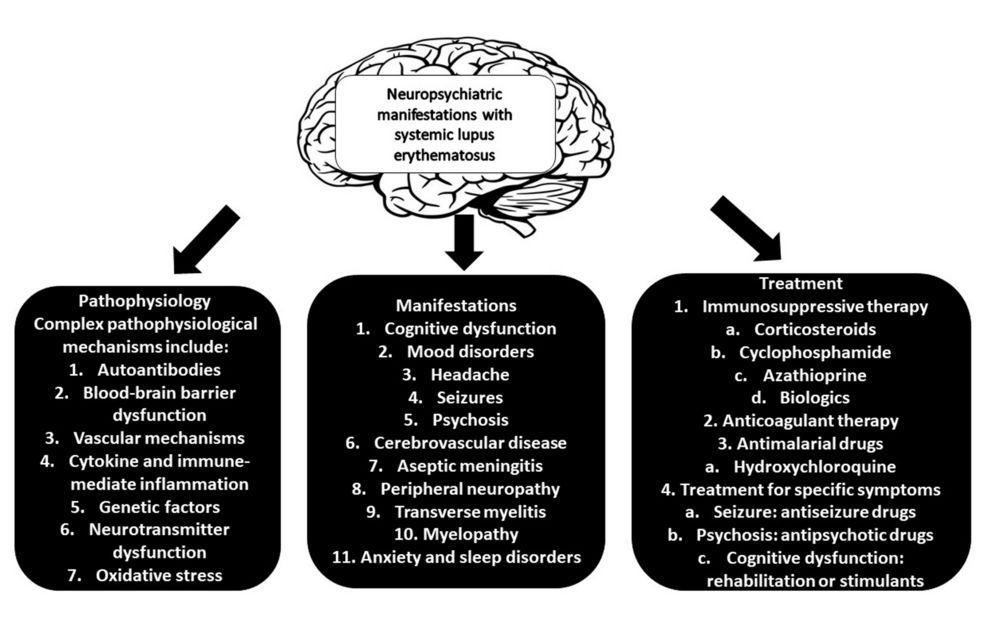
Full read review of Neuropsychiatric SLE. NPSLE criteria includes 12 central & 7 peripheral findings (psych, cognitive, Sz, CVA/TIA, neuropathy, MS-like). NPSLE AutoAbs are many: Abs against APL, LAC, RP, NMDA, NMO/AQP4, EC, SBSN, UCH-L1, TP1, GAPDH, MAP2, U1RNP https://t.co/HvDX8wSuoB
Dr. John Cush RheumNow ( View Tweet)

Keys to Mastery (5.2.2025)
Dr. Jack Cush reviews the news, articles and drug approvals from the past week on RheumNow.This podcast marks the beginning of our Lupus Campaign called "Lupus Unlocked: Keys to Mastery". This month's campaign on Lupus is sponsored by Aurinia. https://t.co/ke0XaJJJX5
Dr. John Cush RheumNow ( View Tweet)
Finish Birth Cohort followed since 1986, who were asymptomatic, found abnormal MRI knee findings, esp cartilage defects in the patellofemoral (56%) & tibiofemoral joints (25%) joints. Small/doubtful patellofemoral (52%) & tibiofemoral (17%) osteophytes seen. Most w/ High BMI. https://t.co/LHqmRHM5wF
Dr. John Cush RheumNow ( View Tweet)
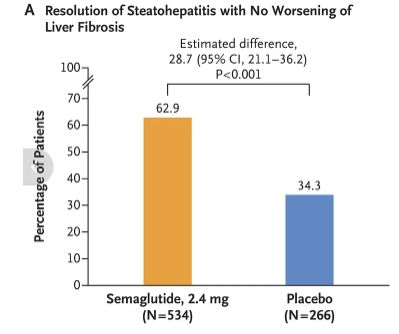
GLP-1 agonists effective in Rxing MASH - metabolic dysfunction-assoc steatohepatitis (AKA NAFLD, NASH). DBRPCT w/ 1100 pts (mean BMI 34-35). A good perspective article from Sensible Medicine. https://t.co/NeaX5qcnPA https://t.co/loRPcwv8B9 https://t.co/ul8ko9cU4K
Links:
Dr. John Cush RheumNow ( View Tweet)