All News
Distinguishing Septic and Gouty Arthritis
A single center, retrospective review of patients undergoing knee joint fluid aspirations for presumed crystalline arthritis (CA) showed that synovial WBC may provide a useful diagnostic marker for SA with an optimal threshold of 50,000 cells/mm3.
Read ArticlePredictors of Developing RA
A prospective cohort study of seropositive arthralgia patients demonstrated that baseline characteristics and labs can be used to predict which arthralgia patients are at highest risk of developing rheumatoid arthritis (RA).
Is High Dose Aspirin Needed in Kawasaki Disease?
Does aspirin add any advantage to intravenous immunoglobulin (IVIG) alone in children with Kawasaki disease (KD)?
Read Article
Rheums! Have a rheumatology question or case for Dr. Cush? Record it here and we may feature it on an upcoming podcast. Tell us your name and where you practice rheumatology.
https://t.co/uLoRmsAkiG https://t.co/ExthuSBSXs
Dr. John Cush RheumNow ( View Tweet)

RheumNow Live 2025 is now available On Demand
For the first time, https://t.co/4UQlqwujiR is offering exclusive access to:
✅ 7 STEP Talks from top rheumatology experts
✅ 21 Powerful lectures and Q & A Panels answering real-world questions
✅ All speaker slides and handouts https://t.co/saW9WH4djO
Links:
Dr. John Cush RheumNow ( View Tweet)
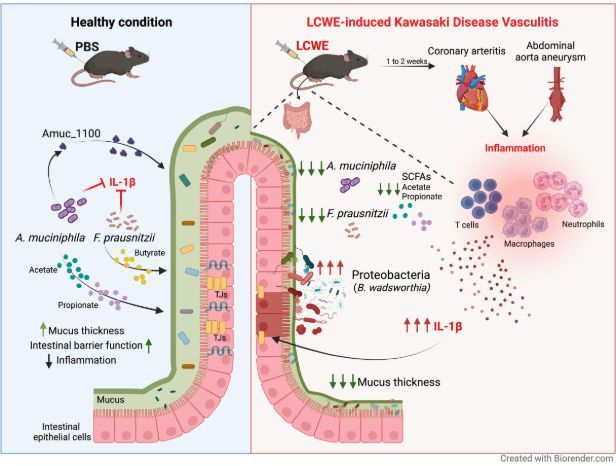
In a murine model of Kawasaki Dz vasculitis- depleting gut microbiota reduces KD like vasculitis. CV lesions were assoc w/ decrease in Akkermansia muciniphila and Faecalibacterium prausnitzii. Replacement attenuated these findings. https://t.co/Hnij8TWkYy https://t.co/wEuVcuAZ5j
Dr. John Cush RheumNow ( View Tweet)

Systematic review of >90 studies on Pregnancy and RA risk indicates that parity, gravidity, infertility, pregnancy loss do not adversely affect RA development. But low birthweight was assoc w/ RA Dx/severity, and pre-eclampsia incr risk of subsequent RA Dx. https://t.co/jVa4tmSqvj
Dr. John Cush RheumNow ( View Tweet)
Is High Dose Aspirin Needed in Kawasaki Disease (KD)?
Does aspirin add any advantage to intravenous immunoglobulin (IVIG) alone in children with KD? A randomized clinical trial IVIG alone was not inferior to IVIG plus aspirin, suggesting that high-dose aspirin during initial https://t.co/vThkP4AH6x
Dr. John Cush RheumNow ( View Tweet)

Recent Medscape Physician Compensation Report 2025, MD Salaries dropped by:
Dermatology: -11%
Neurology: -6%
Urology: -6%
Plastic surgery: -4%
Cardiology: -4%
Ophthalmology: -4%
Gastroenterology: -3%
Rheumatology: -3% https://t.co/WZDM7XxeEU https://t.co/PhRlHaXsMb
Dr. John Cush RheumNow ( View Tweet)
The IL-17A inhibitor, secukinumab, is in late-stage development forthe treatment of giant cell arteritis and polymyalgia rheumatica. https://t.co/JzzqS7bSjd https://t.co/9Qm0YsJmOL
Dr. John Cush RheumNow ( View Tweet)
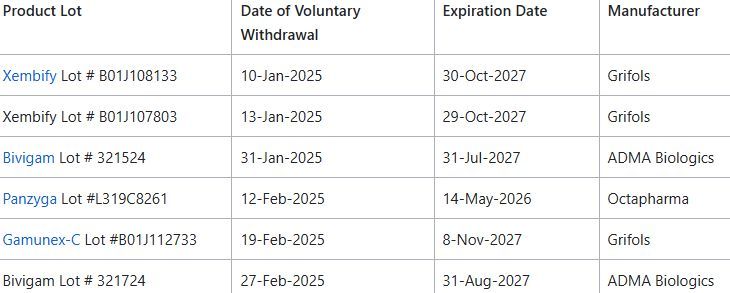
FDA reports higher rate of allergic/hypersensitivity type reactions w/ certainlots of Immune Globulin Intravenous (IGIV) and Immune Globulin Subcutaneous (IGSC) ; these have voluntarily withdrawn by the manufacturers https://t.co/j0yVBodLtW https://t.co/k7s4LtLnyu
Dr. John Cush RheumNow ( View Tweet)
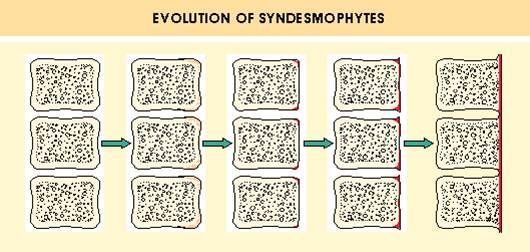
Full read state of the art review of treatments for Axial Spondyloarthritis
Concluding unmet needs:
-Suboptimal Tx Response
- Limited Drug Options
- No Biomarkers
- Extra-Axial Dz
- Non-Inflammatory Pain https://t.co/K3oqRCgTCP https://t.co/rkX2givwF8
Dr. John Cush RheumNow ( View Tweet)
AutoAbs & protein signatures evolve as pts go from Preclinical to established RA. Pts vs controls distinguished w/in 5 yrs of Dx best by iCCP3, RFIgA, RFIgM; and 104 proteins differentially expressed & gene analyses showed 21 pathways enriched ≤5 years of Dx. https://t.co/EWwpvrduJz
Dr. John Cush RheumNow ( View Tweet)
Should Lupus Nephritis Receive PJP Prophylaxis?
A current review article suggests that the need for Pneumocystis jirovecii pneumonia (PJP) prophylaxis in patients with systemic lupus erythematosus (SLE) and lupus nephritis will need to be individualized based on therapies and https://t.co/zzRIVG0Sq3
Dr. John Cush RheumNow ( View Tweet)

#JIA #biologics and #tsDMARDs: Are all patients getting the same access? MDTs, share your insights on on-label and off-label prescribing and help improve future care.
💬 Take the survey by 15 April: https://t.co/vC2Pv5vmH4 https://t.co/rRM5rH0QQl
Links:
BSR RheumatologyUK ( View Tweet)

🚨 New publication alert!
We review the latest advances in imaging for axial spondyloarthritis—from high-res MRI and synthetic CT to deep learning algorithms improving lesion detection.
🔗 https://t.co/oddjoFOGzM
#AxSpA #AIinMedicine
@XBaraliakos @WalterMaks @krystelaouad https://t.co/VwuUwYL1Xs
Links:
Nelly ZIADE 🍀 Nellziade ( View Tweet)
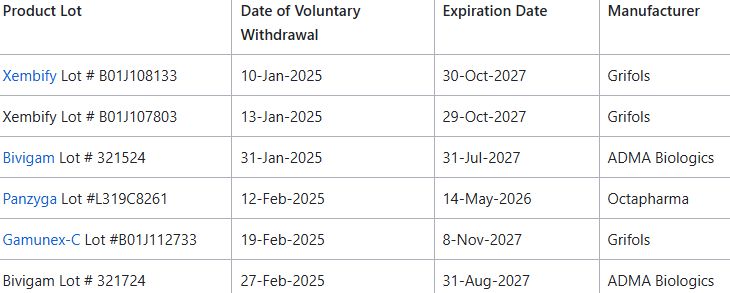
FDA reports higher rate of allergic/hypersensitivity type reactions w/ certainlots of Immune Globulin Intravenous (IGIV) and Immune Globulin Subcutaneous (IGSC) ; these have voluntarily withdrawn by the manufacturers https://t.co/N73Cc8rfAS https://t.co/OTROyomvv8
Dr. John Cush RheumNow ( View Tweet)
Full read state of the art review of treatments for Axial Spondyloarthritis
Concluding unmet needs:
-Suboptimal Tx Response
- Limited Drug Options
- No Biomarkers
- Extra-Axial Dz
- Non-Inflammatory Pain https://t.co/K3oqRCgTCP https://t.co/IPn09jMFM2
Dr. John Cush RheumNow ( View Tweet)

Pregnancy-Related Deaths in the US
A CDC cross-sectional national study showed an increase in pregnancy-related deaths in the US from 2018 to 2022, with highest rates among American Indian, Alaska Native women, and non-Hispanic Black women.
https://t.co/H1s649GyiL https://t.co/MXnrQrAcrT
Dr. John Cush RheumNow ( View Tweet)

ACP: Best Practice Advice on Cannabis or Cannabinoids Use for Chronic Noncancer Pain
The American College of Physicians published a best practice advisory on cannabis or cannabinoids in the Annals of Internal Medicine.
https://t.co/B0eWstjN0Z https://t.co/IzSEIeosme
Dr. John Cush RheumNow ( View Tweet)